People love coining terms to categorize periods of human culture into distinct Ages. I suppose it’s an exercise in how future societies will view us. The industrial age, the computer age, the hydrocarbon age, the information age…even geologists have got into the game, with the term Anthropocene. I wonder if the concrete age might be an apt term, given the millions of crumbling concrete structures we will leave behind. Starting thousands of years ago, we have made increasing use of concrete, to the point that today, especially with rapid third world development, it is having a significant environmental impact, and not in a good way. In this article I will take a look at concrete’s chemistry, history, environmental impact, and will finish by touching on wood products as a concrete alternative.
Cement, composition, clinker, chemistry, creation…
Let’s start by clearing up the difference between concrete and cement. Concrete is a mixture of several materials, usually aggregates held together by cement. So in the context of concrete, cement is the bonding agent that holds together all its other constituents.
Throughout history humans have developed and used several types of cement – lime, gypsum, calcined lime, pozzolanic lime, etc. Since its development in 1824 by Joseph Aspdin, Portland cement has become the cement used in most concretes, so that is what will be focused on in this article, but there are other types of cement used depending on the required properties. In the US five types of Portland cement are classified, designated by Portland I, II, III, IV, and IV, and most developed countries have similar classification systems. Within each category are more specific types of cement. The differences are found in their components, which are chosen by type and proportion to determine two primary properties - set time and resistance to sulfate attack – and many other secondary properties, including resistance to frost damage, even setting, high or low viscosity, high or low bonding strength, resistance to leaching, tendency to inhibit rebar corrosion, etc.
Composition
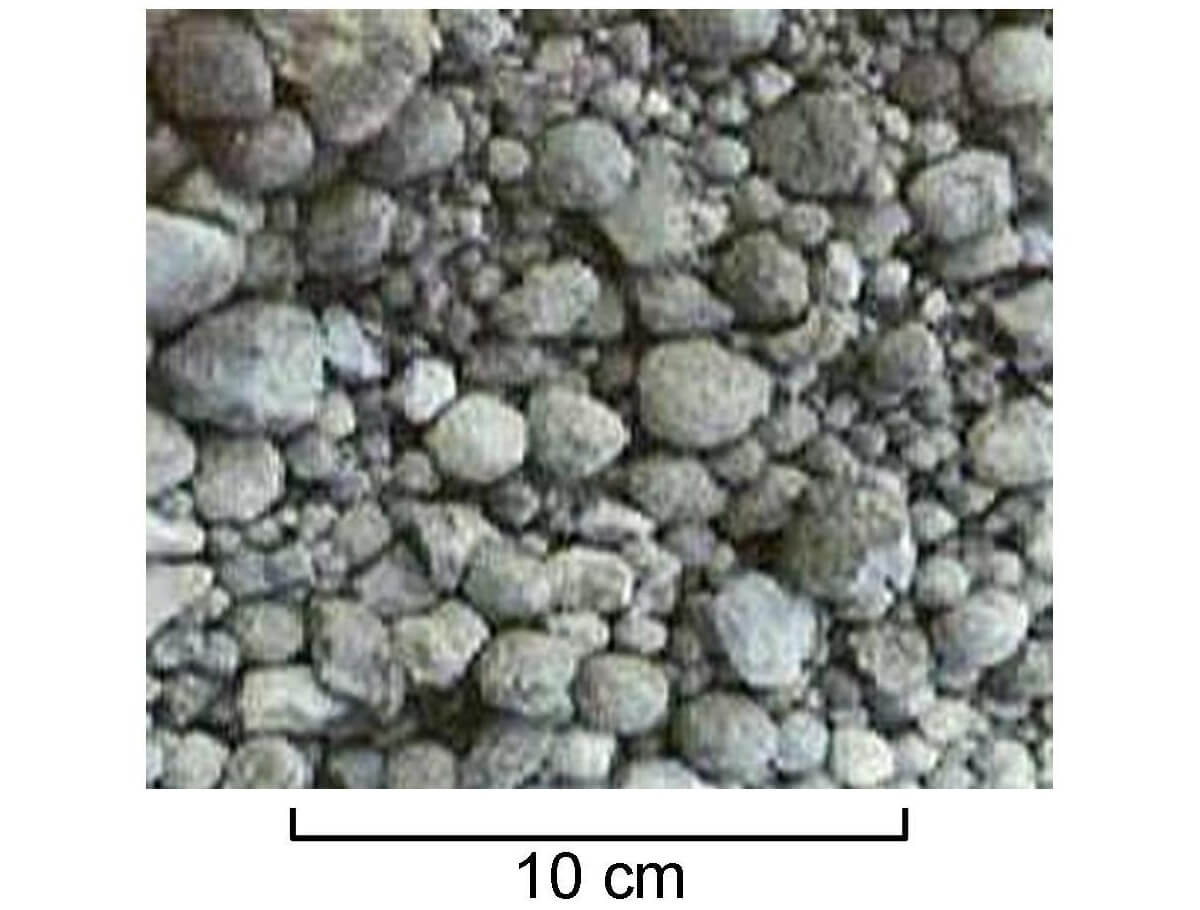
Portland cement is made up of four basic ingredients: calcium, clay, silicon and iron. These constituents are ground to a fine powder, thoroughly mixed, then put into a rotary kiln which is heated. When the kiln reaches sintering temperature at ~1450 C, a chemical reaction begins, creating what is known as clinker - calcium silicate nodules a few mm in diameter (Figure 1). Figure 2 shows a typical cement kiln, with the long tube being the rotary kiln, the tires are the two rings at either end of the tube, and the drive mechanism is the more central ring housed within the circular sheet metal box; the heat source can’t be seen but is typically a gas-fed burner that projects heat and flames into one end of the tube.
The four basic ingredients can obviously be found in many types of natural and manmade materials, and several factors are considered when a cement manufacturer sources these ingredients, including chemistry, cost, proximity, purity, whether it will require a lot of crushing and grinding (like limestone) or little (like clay), and so on. Typically, the mixture put into the kiln is mainly crushed limestone, plus some alumino-silicate clays. Often the limestone used already contains a significant amount of clay, reducing the amount that needs to be added. Secondary additives can include bauxite, sand, iron ore, slag, and fly ash; aluminum and iron oxides can also be added as fluxes which reduce the sintering temperature.
Clinker is easily stored and transported, and has a long shelf life, therefore it is a global commodity traded in high volumes. The creation of clinker, involving significant grinding, crushing and heating, is very energy intensive, and it is estimated that clinker production generates ~8% of global manmade carbon emissions. It is a villain of sorts often overlooked in climate change debates. Cement manufacturers are of course aware of their environmental footprint and are continually finding ways to reduce the amount of energy required. One big step forward has been in the development of dry milling processes; in former times cement constituents were crushed and ground into powder in a wet slurry, which meant the water had to be burned off at great energy expense. Calgary readers will be familiar with Lafarge’s cement plant in Exshaw; it announced an emissions reduction project in early 2019 which is subsidized by the government of Alberta (cbc.ca, 2019).
To create cement from clinker is fairly simple – the clinker is ground to a fine powder. To then create concrete, the cement is mixed with aggregate and water. Ready mix concrete, which is what is most commonly sold in home handyman stores, comes in bags with the aggregates already mixed in. Commercial users and producers of concrete usually buy the aggregates themselves in bulk as this is cheaper. There is a common misconception that it is the water that starts a chemical reaction, and then it needs to evaporate quickly in order for the hard concrete to form. In fact the chemistry of the process, known as hydration, is a bit more complicated than that, and can be broken into two main stages, dissolution and precipitation.

Cement chemistry
First, when the water is added, the powdered cement constituents give off ions and quickly dissolve in the water. The silicates – tricalcium silicate (3 CaO.SiO2 or C3S), dicalcium silicate (2 CaO.SiO2 or C2S) – and the tetracalcium alumino-ferrite (4 CaO.Al2O3.Fe2O3, or C4AF) are very soluble, and the tricalcium aluminate (3 CaO.Al2O3, or C3A) is extremely soluble. The ionic concentrations increase rapidly in the pore solution, until supersaturation is reached, and the second main stage, precipitation, begins.
At this point the ions start to precipitate into new solid phases which are stable at the prevailing temperature / pressure conditions. The basic science behind this is governed by thermodynamic equilibrium principles, something those of us who survived courses such as igneous petrology will painfully remember. One way to look at it is that the chemical structures created during the kilning process are stable at those high temperatures around 1500ºC. Stability in this context is defined by low free energy, where free energy can be thought of as the combined chemical and thermal energy contained by the phase. Once the cement dissolves in the water at much lower temperatures, its now very unstable constituents are free to form new, stable structures, and this is what happens. The solution essentially provides a transitional bridge between the two different temperature dependent solid phases.
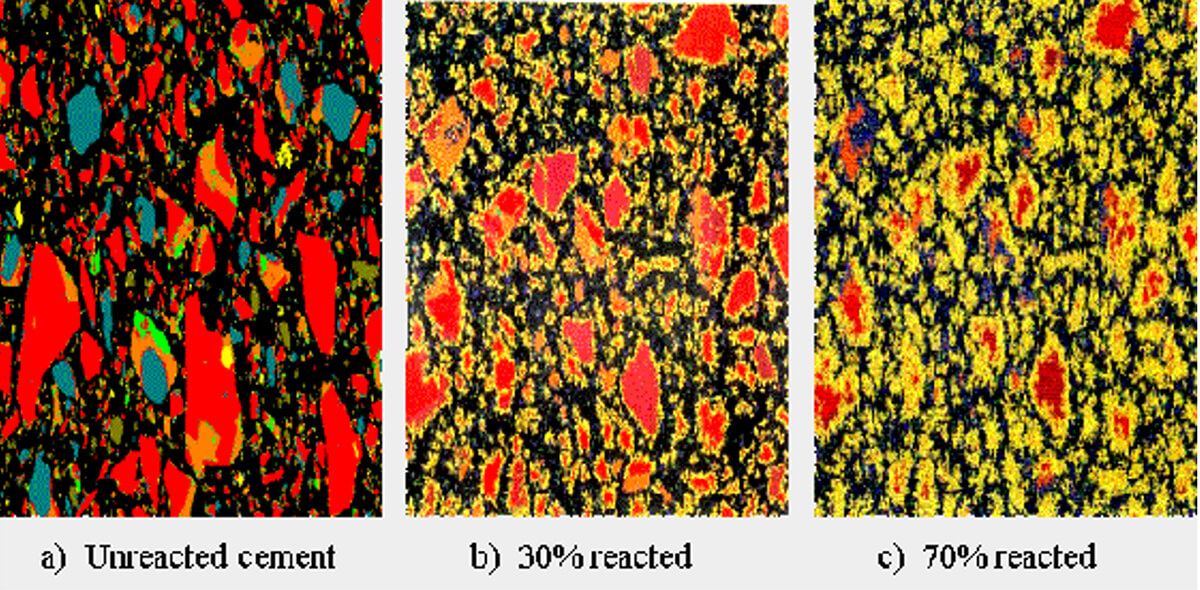
What are the new solid phases? Before diving into that, it should be emphasized that while the individual solid component phases of concrete can be identified and discussed, the hardened cement itself is a quasi-homogenous mass with all solid phases mixed – Figure 3 shows thin section images of cement at various stages of hydration, and this gives a good idea of what the actual morphology looks like. The most important solid phase formed during precipitation is called C-S-H gel, or calcium silicate hydrate. It makes up ~50% of the final solid-state cement and provides most of the strength in concrete. The word gel suggests that it is not a solid, but it is, and most closely resembles the mineral tobermorite. The chemical reaction of its formation from the cement pore solution is:
2Ca3SiO5 + 7H2O → 3CaO · 2SiO2 · 4H2O + 3Ca(OH)2 + 173.6 kJ
As the C-S-H gel precipitates, its form takes on an extremely fine micro-pore structure, and these tiny pores lock in the water given off by the reaction. It does not form into discrete crystal-like structures, rather it forms one entirely continuous layer. The volume occupied by the gel is slightly greater than that of the minerals it replaces, so as it forms it spreads outwards, thus binding together all constituents into one solid block, and is therefore largely responsible for giving concrete its unique monolithic structure. The extra volume it takes up causes any would be capillary pore spaces in the solid mix to be squeezed down to small, isolated pores, thus reducing the overall permeability of the concrete, a desirable characteristic.
The other main solid phases form as follows. The calcium silicates turn into a calcium hydroxide mineral, CH, known as portlandite. The calcium aluminate/ferrite mineral reactions are more complicated, and with very interesting ramifications. If C3A were allowed to react with pure water, a two-stage reaction ultimately ending in the formation of hydrogarnet (C3AH6) would occur; the initial stage of this reaction is extremely fast and could create what is known as flash setting, an obviously undesirable tendency in concrete which usually requires some time to transport and pour. Because of this, gypsum is always a Portland cement component; it is as soluble as the C3A, and the calcium and sulphur ions it releases into solution cause the C3A to follow a different reaction, creating the mineral ettringite (C6AS3H32). If this reaction runs out of sulphur, which is what happens by design, then the ettringite in turn reacts with the remaining C3A and C4AF to form monosulfoaluminate (C4ASH12).
A properly set Portland cement-based concrete will thus be a solid made up of a largely homogenous and hard mix of C-S-H, portlandite and monosulfoaluminate binding together the aggregates. However, if the concrete is exposed later on to a source of sulphur ions, such as ground water, then the monosulfoaluminate will react with the sulphur to create ettringite once again. This is very dangerous as ettringite is higher volume than the monosulfoaluminate it replaces, and therefore the concrete will expand, crack unpredictably, and could suffer catastrophic failure. The primary way to prevent this is to achieve low permeability within the hardened cement so sulphur-bearing fluids simply can’t get in. This is mainly done in two ways: by ensuring that the C-S-H gel fully forms and squeezes capillary pores as discussed above, and by keeping water content as low as possible – the volume of capillary pore space available in the solution is directly related to the relative water content, therefore lower water content equals fewer and smaller capillary pores, equals lower permeability.
In terms of timing, the dissolution stage usually takes less than a minute; it is very fast. A short – perhaps an hour or two – and poorly understood induction period then follows where no precipitation occurs. This induction period represents one of concrete’s greatest advantages – that it does not set immediately. That one to two hours while the concrete is in a liquid slurry form is more than enough time to transport, optionally pump, and pour it. Next comes a period of fast initial precipitation that lasts about one day, where it is mainly the C3A hydrating. After this, precipitation really slows down, as water content drops and the other alumino-silicates go through the hydration process - some concretes never fully hydrate. During this period precipitation really depends on the ability of ions and water to find each other, something governed by diffusion processes.
One potential problem with concrete occurs if setting takes place at different rates, especially if the concrete sets too fast on the outside relative to the inside. In this case as the setting exterior sheds excess water its volume is reduced, it can no longer fully envelope the still-to-set interior, and cracks appear. Obviously, this is undesirable, and so various methods are used to prevent it. Ideally, the very minimum of water is used, only enough for the liquid concrete mixture to flow and fill the space it needs to occupy. Sometimes you will see concrete workers, especially in very hot and dry conditions, spraying water on setting concrete, and this is to slow down the setting process on the surface to prevent cracking. Concrete doesn’t need exposure to air to set, and actually sets better when poured in underwater environments where evaporation doesn’t create the potential for the exterior skin to set too fast.
Aggregate
A typical concrete is made up of the following rough proportions:
Aggregate: 60%-80%
Water: 14%-18%
Cement: 10%-15%
Air: 2%-8%
As a largely inert component of concrete that plays little or no structural role other than taking up space, the aggregate found in concrete is not usually given much attention. However since it makes up the greatest volume of the mix, it can greatly affect its performance and cost. Aggregate is much cheaper than cement, so the greater the proportion of aggregate, the lower the cost. Availability is another important factor – often there are only certain types of aggregate available, or the costs of getting something ideal are prohibitively high. A finer aggregate will be more workable and provide a smoother finish, but a coarser aggregate costs less. Often most of a structure will be built with a coarse concrete, then finished off with a very fine-grained concrete for cosmetic reasons. Desirable aggregate qualities include a tendency not to crumble (not friable), consistent size and hardness, low porosity and permeability, and resistance to weathering.
History
The Romans are often credited with inventing concrete, but primitive forms of it were in use far earlier. All sorts of places are mentioned as being the first or early users of concrete, including the Nabataeans of the Middle East who used concrete around 2700 BP (before present), not only in buildings, but also in their sophisticated, for the time, water management systems. It is believed that as early as 8500 BP ancient Syrians discovered lime and its unique binding properties when they experimented with the calcined lime formed on the surfaces of the limestone-lined fire pits they used for cooking and heating. Other early examples of concrete-like materials can be seen in classical Greece, ancient parts of Europe, the Egyptian pyramids, and parts of the Great Wall of China. One can safely say that like many other human technologies, concrete was probably developed independently in many places and at different times around the world, and that there were also technology transfers between different cultures via conquest, imitation, movement of experts for hire, etc. There is general agreement though that the Romans were the first culture to really take concrete to an industrial scale, and to systematically experiment with it and develop different types for different purposes.
The term Roman concrete (or opus caementicium in Latin) actually refers to a specific type of concrete that they developed sometime between 2250 BP and 2150 BC, and which represented a big advance that made the building spree that followed possible. The key addition to the basic ingredients was a powdery volcanic sand referred to as pozzolana, or pulvis puteolanus in Latin. There were two main sources for it, one near Naples, the other near Rome. It makes me wonder whether this proximity was a factor in Rome and Naples developing into two of Italy’s biggest cities. Pozzolana tends to contain a mineral called phillipsite, part of the zeolite group and rich in aluminum. If seawater was allowed to percolate through the concrete, it caused a reaction with the phillipsite which resulted in the formation of tobermorite, which as mentioned above is similar to portlandite, making the Roman concrete extremely strong and durable. This development led the Romans to use concrete in underwater settings, with perhaps the most famous example being Caesarea, now an archaeological site in modern day Israel. Vitruvius wrote in 1965 BP that the best ratio for above water cement was 1:3 parts lime to pozzolana, and for underwater cement 1:2, which interestingly enough are basically the same ratios used today.
A brief aside on modern concretes, which tend to be damaged by exposure to salt. While the mildly acidic nature of any type of salt will attack the chemical bonds within a concrete’s cement, it is the water which salt draws into the concrete’s pores which does the most damage. Salt attracts and holds up to 10% more water in the pores, which then expands and contracts during freeze/thaw cycles, resulting in the commonly seen surface damage called spalling. More serious from a structural perspective, salt causes carbonation, which lowers the pH of the concrete and accelerates the corrosion of steel rebar. In Toronto, which is typical for a large cold winter North American city, 10,000 – 12,000 tons of salt, or 100 Kg per lane km, are put on the roads each winter storm (Knope, 2019), accelerating the deterioration of the concrete backbone of its highway system (not to mention long term damage to its soils and waterways). One can only imagine the ultimate cost of this strategy, in terms of concrete repairs and replacement, but anyone currently driving the 401 highway can see all the corroded rebar and rotten concrete exposed by hundreds of bridge and overpass repairs across the GTA.
Historians refer to the period that followed the development of Roman concrete as the “concrete revolution”. Throughout their empire the Romans built all the structures whose remains we marvel at – domes, amphitheatres, dams, palaces, bridges, etc. The Pantheon in Rome still stands as the world’s biggest unreinforced concrete dome, and it was built almost 2,000 years ago (Figure 4)! The longevity of Roman concrete is in evidence throughout Europe, North Africa and the Middle East, whereas modern concretes typically last about 100 years. As the Roman empire declined, concrete expertise faded, and Europe entered a period where construction reverted to older methods using wood and stone. While concrete was still in use throughout the Middle Ages and Renaissance (i.e. between the end of the Roman empire and the end of European Age of Enlightenment, roughly 476 – 1815 AD) it was not used as often as during the Roman period and tended to be of lower quality. Almost all of Europe’s famous Middle Age churches were built with stone, not concrete. In 1414 instructions for making pozzolan cement were rediscovered and this started a slow readoption of concrete use in Europe. In 1755, frustrated by structural failures in the Eddystone Lighthouse, a British Engineer named John Smeaton experimented with cement mixtures and was involved in the development of the clinker process. This kick started modern concrete improvements, including the very significant development of reinforced concrete – essentially embedding structural components with high tensile and ductile strength, such as steel rebar, to compensate for concrete’s low tensile and ductile strength – which brought us to the modern concretes used all over the world today.

Environmental impact
To give CSEG members employed in the oil & gas sector a bit of a break from being environmentalists’ societal whipping boys, I thought I’d end with a section on the significant environmental cost that is being paid for the extensive use of concrete around the world. Currently, annual global concrete production is above 4 billion tonnes! While concrete is more or less chemically inert and does not pose direct health risks, one could argue that its production is slowly and inexorably killing our planet.
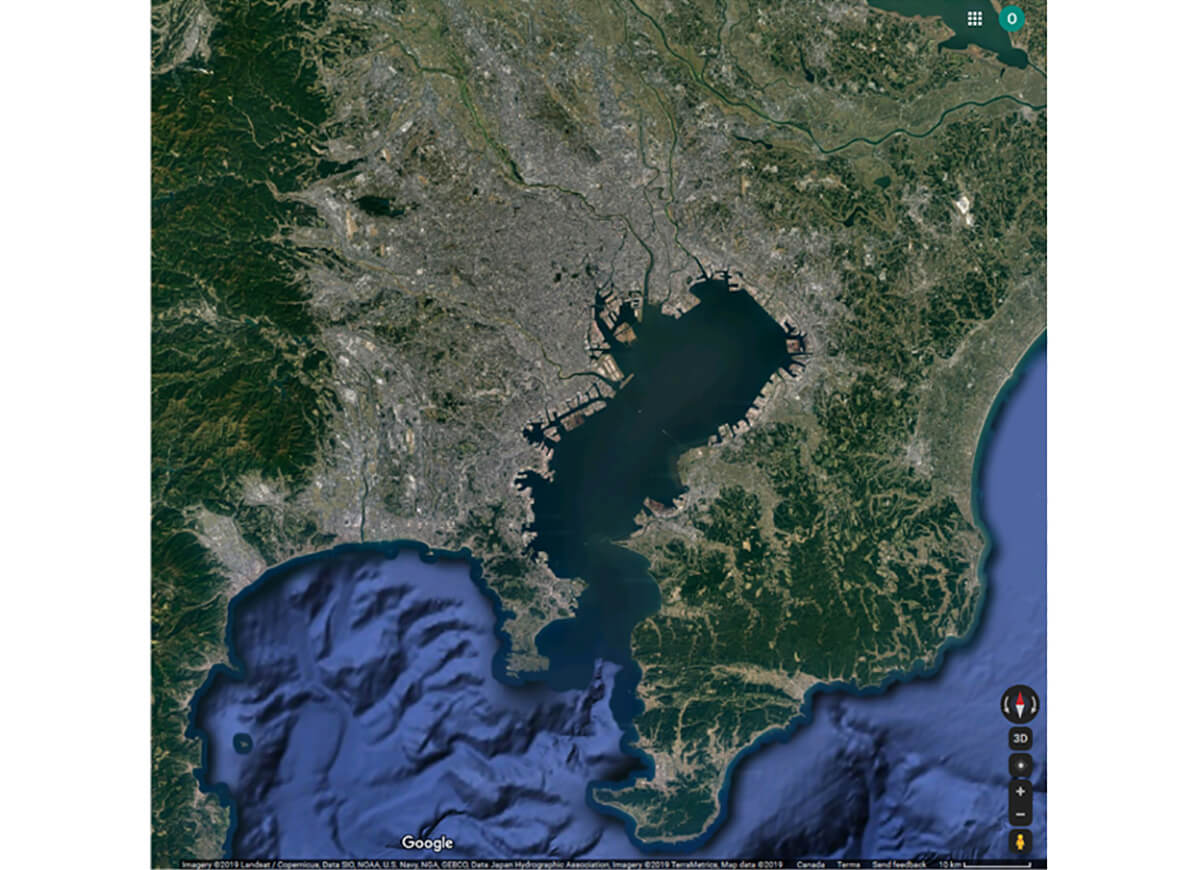
About 10% of the world’s industrial water use goes towards concrete production, but the impact is greater than the numbers suggest because 75% of this water is being used in parts of the world where water is in short supply. Much of that water stays locked in the concrete. As previously mentioned, up to 8% of the world’s manmade CO2 emissions come from concrete production, mostly from clinker kilns. Concrete construction and limestone, sand and gravel quarries create a lot of dust and air pollution. The energy used and pollution caused by simply transporting concrete is significant. The mining of sand destined for use in concrete has become a global problem, with many of the world’s beaches and river deltas being dredged and dug up, often illegally, permanently removing valuable ecosystems. Worst of all, many of the world’s most fertile and life sustaining-areas are gradually being paved over with concrete, essentially blocking the ability of the earth to absorb and give off air and water, and to generally sustain nature in all its complexity. Simply look at a large modern city such as Tokyo on Google Maps – thousands of square kilometres surrounding Tokyo Bay, which not much more than a hundred years ago supported a vibrant and beautiful natural ecosystem, are now just the grey colour of concrete, choked of most life other than humans (Figure 5).
Unlike stone and Roman concrete structures, modern concrete ones will all need to be replaced, meaning everything built with concrete during the post WWII boom will soon be nearing end of life, and require replacement and or repair. The time for public debate and decisions based on longer-term thinking is now. Do we simply want to replace concrete with more concrete with all its costs? Or do we want to develop and phase in new, more environmentally friendly materials? One encouraging trend is the use of modern, wood-based structural materials in place of reinforced concrete. The rapidly developing field of mass timber construction, as it’s known, employs several types of wood products, including glue-laminated timber (glulam), cross-laminated timber (CLT) and nail-laminated lumber (NLT) that rival or exceed steel and reinforced concrete in terms of strength, weight, cost and workability. While not totally eliminating the use of steel and concrete, this type of construction greatly reduces it, therefore providing much lower energy costs. While not widely acknowledged, wood is one of the most effective carbon capture mechanisms known. Each kilogram of a typical tree’s growth takes 1.55 Kg of CO2 out of the atmosphere, and that is basically locked in the wood until it rots or burns.
Figure 6 shows a recent example, Carbon12, a condo building in Portland designed by a cousin of mine, Ben Kaiser. Not only is it a beautiful, environmentally friendly building, it is more earthquake proof than a reinforced concrete building, perfect for a seismically active place such as Portland. Ben tells me his wood buildings are approximately 20% of the weight of concrete/steel equivalents. On a recent project of his, the contract included a pledge, “to plant the trees equal in board footage (at maturity) used in [our] buildings prior to receiving an occupancy permit. This will close the loop in regard to known line of custody and carbon sequestration.” Perhaps this is the sort of model that will allow humans to become better citizens of the Earth.











Share This Column