Several months ago when I was picking this topic, opioids were arguably considered the biggest current health crisis, but of course now that has been eclipsed by COVID-19. Brian Schulte (RECORDER editor) and I considered switching the topic to COVID-19, but decided against it, since the news is swamped with details about the virus and we’ve all no doubt become experts. The pandemic will come and go, but society’s dangerous addiction to opioids will remain. And I’m sure COVID-19 related stresses have made the opioid crisis worse, as people lose their jobs and their lives are otherwise disrupted in many ways.
Just recently I heard of a tragic example of this. A relative of mine by marriage, a young woman in her 20’s with some promise in life, died of a fentanyl overdose. She had been in rehab trying to get her life back on track when COVID-19 forced the clinic to temporarily shut down. She returned home to Florida to stay with her parents. One day she was doing laundry in the basement and chatting with them as she went back and forth from her bedroom between loads. After a while they realised they hadn’t seen her in a while, and not getting a response from her locked door, kicked the lock open to find her slumped dead in bed. I found this disturbing, not because I knew her (I didn’t), but rather because of that everyday detail of doing the laundry. It brought it much closer to home as a crisis that is forcing its tragedies onto families that could be our own. In this article I will cover the two aspects I am most interested in – the history of the various opioids, and the reasons why they are so addictive, causing otherwise rational humans to willingly make life choices leading to misery and likely premature death.
History
The term opioid refers to substances that will bind to opioid receptors in the human body. This includes endogenous (originating internally) opioids such as endorphins, and exogenous (originating externally) opioids such as opium. The term opiate is an old one and refers to opioids that are derived from the opium poppy (Papaver somniferum, Fig. 1). Therefore, opiates are a subset of the exogenous opioids, which now include many partially synthetic (e.g. heroin) or fully synthetic (e.g. fentanyl) opioids which have been developed much more recently than the poppy-derived ones.
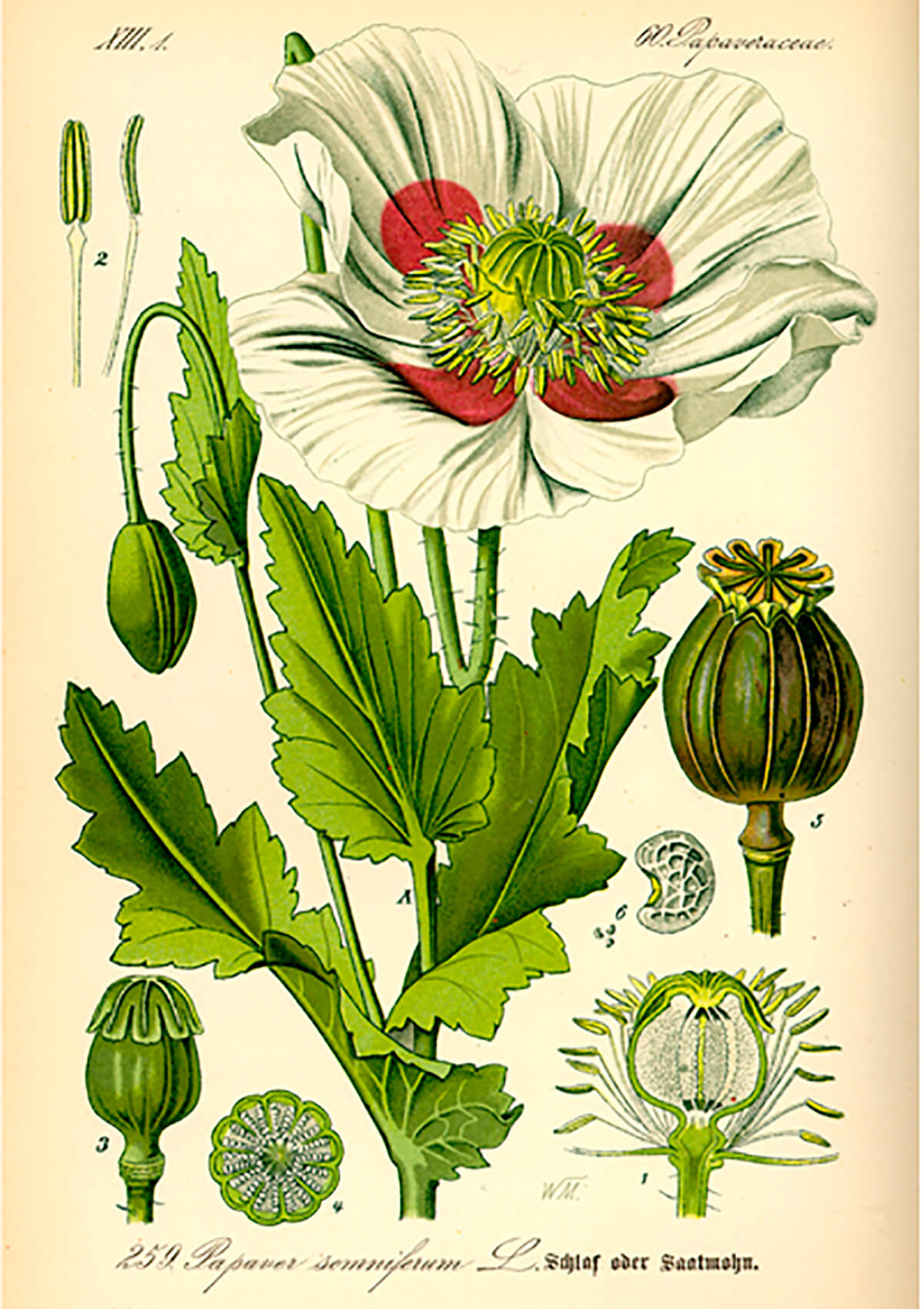
Scientists believe that opioid receptors first appeared in vertebrates approximately 450 million years ago and have since then evolved and become central to behavioral control systems in all vertebrates. This is the critical aspect of everything related to opioids – they are the messengers used by ancient and fundamental behavioral systems. Exogenous opiates are hijacking complex systems that are at the very centre of who we are and how we feel about and react to things.
My initial research indicated that the first evidence of people using opium comes from Sumeria around 3400 BC. Supposedly they cultivated the opium poppy and called it hul gil, or “joy plant”. Then I came across a quite convincing debunking (Breen, 2018). Breen argues that this is a, “great example of how historical misinformation spreads even long after it has been proven wrong.” It appears the now widespread assertion that Sumerians used opium was based on a travel book from 1914, written by a British colonial doctor, A.R. Neligan. He had spoken with a British archaeologist, who speculated that an Assyrian word he’d found in a text from around the 7th century BC might have Sumerian roots and might refer to opium since it could be a compound noun combining “joy” and “plant”.
Subsequently archaeologists jumped on this and concluded that plants depicted in Sumerian bas reliefs were poppies, but I agree with Breen that they look much more like pomegranates (Fig. 2), a very common graphical motif in that region.

Regardless, it’s safe to say poppy cultivation and the use of opium started in the Middle East at least 3300 years ago, as there is proof of that in Egypt around that time, and in Cyprus some 200 years later. Opium use spread from the Middle East throughout Eurasia and it was imbibed for a wide range of medicinal purposes. In the 1500’s Paracelsus, the Swiss alchemist/philosopher, created some kind of medicinal concoction he called laudanum which may or may not have contained opium. In 1676 the English physician Thomas Sydenham produced a substance which definitely did contain opium, and he also called it laudanum. That started a period where various opium-based mixtures, usually in an alcohol solution and known by the generic term laudanum, were used to treat almost every type of ailment in Europe, of which there was no shortage given the often wretched, unsanitary conditions they lived in. These remedies really must have seemed like miracle drugs given that they alleviated a broad range of symptoms including coughing, cramps, pain, and diarrhea, all the while delivering a pleasant, light headed and dreamy feeling.
In 1803 the extraction of morphine from opium was achieved in Germany. This was the most purified form of opioid produced to date, and therefore much more potent than those before it, which would look like crude herbal remedies in comparison. The 1900’s saw many clinical opioid developments involving opium products, with key milestones including the extraction of codeine in 1830 by Frenchman Jean-Pierre Robiquet, and synthetization (via acetylation of morphine) of heroin in 1874. Ironically, the chemists who developed heroin were trying to find a painkiller less addictive than morphine, but it turned out to be twice as effective as a painkiller and twice as addictive as well. In 1898 Bayer began commercial production of heroin. Throughout the 1800’s, while the pain reduction benefits of these powerful new opioids were appreciated, their destructive addictive properties were also becoming obvious. This double-edged sword is still with us today – we want the pain relief opioids give us but struggle to deal with the social costs that opioid addiction also creates.
Opioid receptor systems
To understand why opioids are so addictive, one really must look at the biochemistry of the body’s opioid systems, and specifically the effect the different opioid receptors have on the brain. There are four main opioid receptor types in humans – delta (δ), kappa (κ), mu (μ), and nociceptin. The following table is derived from (Wikimedia Foundation Inc., 2020).
| Receptor type | Location of receptors within body | Function |
|---|---|---|
| Table 1. Summary of major human opioid receptors, adapted from (Wikimedia Foundation Inc., 2020). | ||
| delta (δ) |
|
|
|
kappa (κ) |
||
|
mu (μ) |
|
μ1: μ2: μ3:
|
|
nociceptin |
|
|
The opioid system is one mechanism our brains use to respond appropriately to experiences detected by our senses and sent to the brain for translation. Species succeed when they do certain things and don’t do other things, and the opioid system is one important way the body ensures this happens. The chain of events starts by the brain translating some specific experience. This triggers the release of specific endogenous opioids, a type of neuropeptide, which are protein-like molecules used to send signals between neurons. In humans there are five types of opioid peptides – enkaphelines, endorphins, dinorphins, nociceptins, and endomorphins. (Note there are other peptides such as oxytocin, and other systems similar to the opioid one.) Once released, the peptides bind to the appropriate receptors, which then stimulate the functions summarized in the table. Let’s use an endorphin example, since it’s endorphins that the synthetic opioids mimic.
Endorphins
Our brains have evolved to perceive certain strenuous, possibly pain-inducing activities – such as sex, or running away from danger – as beneficial, so endorphins are released. There are several types of endorphin molecules; they are classified as endorphins based on their function, not their molecular structures which are quite different from one to the next. They are all produced in the pituitary gland and all bind primarily to the mu receptors, which, referring to the table above, are associated with a number of functions but most interestingly, pain reduction and euphoria. So we simultaneously experience a reduction or absence of pain (which otherwise would have likely caused us to stop the activity), and a sense of euphoria (which makes us want to continue the activity, and do it again in the future).
I should point out that pain reduction and euphoria within the opioid system are probably one and the same – the state of euphoria should probably not be viewed as a side effect, but rather an extremely effective way to reduce the experience of pain. Remember, pain is not something tangible, it is something created by the brain, and by the same token the brain can create something different and stronger that drowns out the pain, resulting in an apparent reduction of pain. Sound is a useful analogy. Let’s say pain is represented by an awful (to our brains) sound like scratching on a blackboard. Instead of trying to stop that sensation, the brain creates something different and really pleasurable, like very loud harp music (maybe not pleasurable to many, but you get my point).
What actually happens at the neurochemical level? The chemistry is extremely complex, but if you look beyond that it is all just a sequence of events at the chemical level: chemical A + chemical B cause chemical C to be released, which in turn acts on chemical D, and so on. First, the pituitary gland produces a precursor neuropeptide protein called proopiomelanocortin (POMC). This relatively complex protein is then broken down into simpler proteins, including beta-lipotropin (β-LPH). The beta-lipotropin is then further broken down into three types of peptides which make up the endorphins - α-endorphin, β-endorphin, and γ-endorphin. In response to pain, the pituitary gland releases the three types of endorphins. The β-endorphins mainly bind to receptors within the peripheral nervous system (PNS), basically all the nerves outside the brain and spinal cord (the central nervous system or CNS), and therefore those most likely to be generating pain signals. Within the PNS a neurotransmitter called substance P plays a central role in transmitting pain signals; when β-endorphins bind to the PNS mu receptors the substance P neurotransmitters are blocked, greatly reducing the pain signals received by the brain. Meanwhile, the CNS is also receiving endorphins at its mu receptors. This causes a different neurotransmitter – gamma-aminobutyric acid (GABA) – to be blocked, which in turn causes dopamine to be produced and released into the body. It is dopamine which creates the sensations of pleasure and euphoria in the brain.
Endorphins are liberally used by the brain to reduce pain and reward other, painless and positive behaviours, such as eating and sleeping. I assume that endorphin release - perhaps of only specific endorphins - is also triggered by non-painful experiences, because in my book eating, sleeping, etc. are not painful, except perhaps on rare occasions of overindulgence at Thanksgiving or Christmas. The literature all seems to focus on the pain reduction function, and not so much on the pleasure reward function. But the opiates and all the recently developed synthetic opioids mimic the mu receptor-binding characteristics of endorphins, both pain and pleasure, and there you have the root cause of the double-edged opioid sword. We have found a way of amping up the natural pain relief system nature has given us, but unfortunately that pain relief system is combined with nature’s primary reward system. So when given mu opioids we are ingesting the most primitive, powerful and fundamental reward that our brains can experience, and so obviously we crave ever more, in an attempt to stay in a permanently euphoric state. We are actually designed to be addicts to our own endorphins, to reward the behaviours that help us survive as a species, but our bodies dole out the endogenous drugs in a controlled, safe way. The problem is that we’ve found a way to similarly reward ourselves with exogenous drugs, without doing those positive (in an evolutionary sense) things, and with no real limits other than our ability to get our hands on more of these drugs. It’s a diabolical bargain we make when we accept opioid pain relief treatment. It’s an interesting apparent confirmation of that deeply ingrained western Judeo-Christian belief that anything pleasurable comes at a cost.
Given that the opioid pain relief + reward system has been around in vertebrates since the Ordovician, I find myself pondering the emotional experiences of animals. There is a prevailing feeling that animals do not experience the same rich spectrum of emotions as us humans, and that when we imagine they do – for example when watching dogs playing in an off leash area – we are guilty of anthropomorphizing, or projecting human characteristics onto animals lacking those qualities. But if all vertebrates have that same euphoria-delivering endorphin reward system, then surely that is not a human characteristic but rather something found throughout the animal kingdom? And all or most of the other basic neurochemical workings of our bodies are probably just as old or older and as widely shared among all animals. So, who is to say that all living creatures with spinal cords are not experiencing those same inner feelings of joy that we humans occasionally feel, as well as all the other emotions? It seems more likely than not that they do, and that certainly makes me feel closer to all those animals and to better appreciate the sanctity of their lives. Note that I am not suggesting that animals experience all the additional layers of complexity that our intellectual, conscious brains pile on to these raw emotions.
Overdoses
But back to opioids and the mess we’ve created for ourselves. What actually happens during an overdose? If synthetic opioids are mimicking nature’s own opioids, why are they harmful? The main reason is that the synthetic opioids are extremely concentrated and therefore triggering much stronger responses in the receptors than those from the body’s own opioid peptides. Below is a table adapted from WHO materials that shows the analgesic potencies of different opioids relative to an equivalent morphine dose.
| Analgesic | Potency relative to Morphine | Duration of action (hours)b |
|---|---|---|
| Table 2. Approximate potency of opioids relative to morphine; PO and immediate-release formulations unless stated otherwise. Used under Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO). (World Health Organization, 2018) | ||
| Codeine Dihydrocodeine |
1/10 | 3-6 |
| Pethidine | 1/8 | 2-4 |
| Tapentadol | 1/3 | 4-6 |
| Hydrocodone (not United Kingdom) |
2/3 | 4-8 |
| Oxycodone | 1.5(2)c | 3-4 |
| Methadone | 5-10d | 8-12 |
| Hydromorphone | 4-5 (5-7.5)d | 4-5 |
| Buprenorphine (SL) | 80 | 6-8 |
| Buprenorphine (TD) | 100 (75-115)c | Formulation dependent (72-168) |
| Fentanyl (TD) | 100 (150)c | 72 |
| a | Multiply dose of opioid in the first column by relative potency in the second column to determine the equivalent dose of morphine sulfate/hydrochloride; conversely, divide morphine dose by the relative potency to determine the equivalent dose of another opioid. |
| b | Dependent in part on severity of pain and on dose; often longer-lasting in very elderly and those with renal impairment. |
| c | The numbers in parenthesis are the manufacturers’ preferred relative potencies. |
| d | A single 5 mg dose of methadone is equivalent to morphine 7.5 mg, but a variable long plasma half-life and broad-spectrum receptor affinity result in a much higher-than-expected relative potency when administered regularly - sometimes much higher than the range given above. Therefore, guidance from a specialist is recommended for conversions to regularly administered methadone. |
Note that morphine is a powerful drug in its own right, and many people have become addicted to it; this was a particular problem among soldiers wounded during the US civil war. Then ask yourself, if fentanyl is 100 to 150 times stronger as a pain killer, how much stronger are the other functional responses to it, and further, how much more addictive and deadly is it (Fig. 3)?

Referring again to the table you will see that both delta and mu receptors are associated with respiratory depression; essentially what happens in most fatal opioid overdoses is that the brain signals to the respiratory system are impaired so badly that the reflexive instinct to breathe fails, resulting in suffocation. As well, many fatal overdoses are caused by comatose drug users suffocating on their own vomit, as most of these drugs are hard on the stomach, especially when combined with alcohol, and the body reflexively tries to expel them. Sadly, this is the situation that ultimately resulted in my own younger brother’s death.
I have noted that there seems to have been a change in societal attitudes towards pain over my lifetime, especially lower level, shorter-term pain associated with medical procedures or temporary illnesses. When I was young the prevailing attitude was that pain was an unavoidable part of these experiences, and something you should try to tough out – the grin and bear it school of thought. Nowadays it seems like prescriptions for opioids are thrust into your hands before you’re even asked if it hurts or not. The attitude seems to be that any level of pain is unacceptable and proactive steps in the form of opioid pain medication should be taken, whether it’s really needed or not. If accurate, this surely has contributed to the opioid crisis, but I realize my personal perspective is just that, and not necessarily generally representative. But many are stating the obvious which is that pain treatment not only benefits the patient, but also the pharmaceutical industry that produces and profits from the opioids, and that surely they have influenced the attitudes of the doctors who prescribe the pain medications. One only has to look at the marketing budgets of the pharmaceutical companies to wonder where it is all being spent. It’s a sad observation that 150 years after heroin was pushed as a drug less addictive than morphine, we see the same story repeated, with the same claims of being less addictive being made about oxycontin, fentanyl, etc., each of which has turned out to be more addictive than the last, and the addiction rates and number of overdoses keep rising.
Editor’s note
In June 2020, for the second consecutive month, British Columbia reported the highest number (175) of illicit drug toxicity deaths predominately caused by fentanyl. The British Columbia government has seen a correlation between the COVID-19 time period and the illicit drug crisis (Johnston, 2020).










Share This Column