
This past summer saw thousands of fireworks displays across Canada as we celebrated 150 years of Confederation. I’m sure few of us really understand how fireworks work, so this article will educate and prepare us for 2067, although if not dead by then I’ll certainly be deaf and blind.
The first documented reference to fireworks is from China during the 7th century Tang Dynasty. To this day, fireworks have played an important role in Chinese culture, and China is currently the world’s largest manufacturer, consumer and exporter of fireworks. Figure 1 is a nice illustration of a Ming Dynasty crowd watching fireworks, taken from an early 1600’s edition of the novel Jin Ping Mei. On a side note, I had a scholarly great uncle, Franz Kuhn (1884-1961), an early translator of many classical Chinese novels, including several containing erotic content such as Jin Ping Mei. Perhaps the ancient Chinese also used fireworks as a visual euphemism for sex? Moving on…

Fireworks gradually spread westward along the Silk Road and other trade routes. The Arabs had gained knowledge of gunpowder use, including fireworks, by the mid-1200’s. Curiously, it took another 500 years or so before fireworks really caught on in Europe. Today, however, they are popular around the globe, and are an integral part of many large festivals and celebrations across a broad range of cultures.
A lot goes into fireworks, so I will first describe their basic components, and then explain how the main effects are achieved. Obviously, I cannot detail all the variations, so instead will describe how a common aerial firework is put together and works. Simple variations on these basic methods easily explain other types of fireworks.
Components
(Image via explainthatstuff.com)
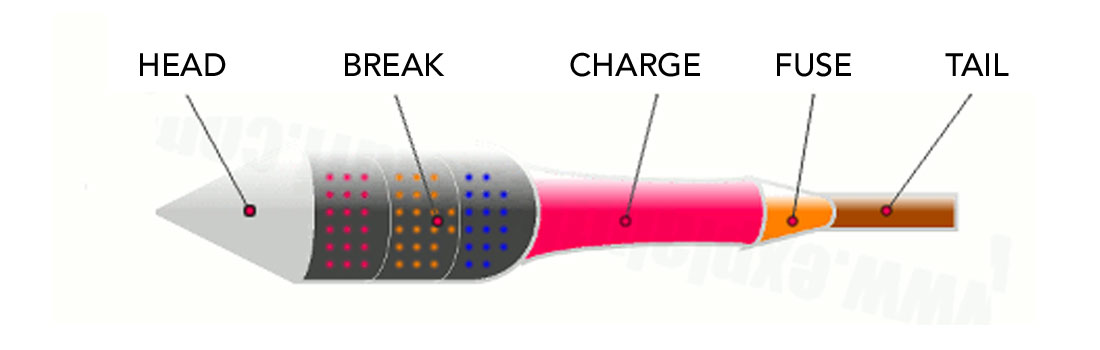
Figure 2 depicts a typical aerial firework rocket. From left to right:
1) The conical head tops off the firework, making it more aerodynamic and straight-flying.
2) The breaks section of the firework contains the compartments with the effects that we see and hear. Each break contains its own effect, and is ignited in sequence via time-delay fuses. Stars is the term given to the key ingredient of the visual effects that are contained inside the breaks, as these are the little chemical concoctions that actually produce the light and colour. You can find more details on these under the ‘Stars’ heading below.
3) The charge is the section of the firework containing the explosive that accelerates the firework to the desired velocity and hence height. It is essentially a pouch of black powder (see more on the explosives used in the ‘Powder’ section below) that burns within a hollow tube of thick cardboard at the base of the firework. The tube is sealed at the top with a clay bulkhead, and at the bottom with a clay nozzle which directs the rocket’s exhaust. The pressure increases as the temperature goes up, until the exhaust begins to shoot out of the nozzle to achieve lift-off!
4) The fuse is the part of the firework that is lit. In most cases there are two fuses which are ignited simultaneously, one fast burning fuse that ignites the charge, and a second slow burning time-delay fuse that ignites the breaks. While the firework ascends, the time-delay fuse continues to burn, and its segments are carefully calibrated so that each effect ignites at the desired height.
5) The tail helps keep the firework’s flight path straight and stable. It also makes it easier for the organizers of the fireworks show to arrange and secure the individual fireworks in the ground.
Note that I have described a rocket style firework above. Many fireworks use a mortar propulsion system rather than a rocket. The two are very similar; with a mortar shell style firework, instead of the break being part of a rocket, it has a lifting charge attached to its base. The lifting charge is a black powder or something similar contained within a thick cardboard sphere. The shell is inserted charge side down into a steel pipe of slightly larger diameter (the mortar). The charge and the shell’s time-delay fuse are simultaneously lit, and the shell shoots up into the air leaving the combusted charge behind. Many over-the-counter fireworks are all-in-one prepackaged mortar-plus-shell fireworks.
Powder
The gunpowder used in fireworks is more accurately known as black powder, and is a simple mixture of sulfur (~10%), charcoal (~15%) and potassium nitrate, also known as saltpeter (~75%). The sulfur and charcoal combine to form the fuel, and the saltpeter acts as an oxidizer. Black powder is a low velocity explosive (161-631 m/sec.), and thus very suitable for fireworks. In other applications, black powder has been eclipsed by more violent explosives such as dynamite, whose chemical reactions are faster and more efficient (e.g. mining explosives have a velocity of 1800-8000 m/sec.). The powder used in fireworks has not changed that much over time. Most fireworks still use black powder, but there are variations, including higher proportions of saltpeter with no sulfur, or entirely different types of powder depending on the purpose. Hybrid fuels are often used for propulsion, producing tail effects such as strobes, sparkles and whistles.
The chemical formula describing the combustion of the black powder is:
2KNO3 (potassium nitrate) + S (sulfur) + 3C (carbon in charcoal form) → K2S (potassium sulfide) + N2 (nitrogen gas) + 3CO2 (carbon dioxide)
Historians generally agree that the Chinese developed black powder in the 9th century, which obviously does not gibe with a 7th century or earlier date for the invention of fireworks. Until the development of black powder, the Chinese used various mixtures of charcoal and sulfur, which would have given off bright flames, smoke and hissing noises – still impressive, but without the bang that adding saltpeter would eventually provide. Lately, progress has been made in the use of compressed air as a replacement for powder; this is mainly driven by a desire for more accurate and predictable explosions that are a necessity for increasingly complex displays. I wonder if there is some cross over between seismic air gun and fireworks technology?
Stars
Stars are small, solid, highly combustible cubes with dimensions between 0.16 – 5.0 cm. A break compartment will be filled with many of these cubes mixed with black powder. When the time-delay fuse reaches the section, the black powder and stars ignite, the temperature rises quickly, the higher pressure ruptures the break’s cardboard walls, and the ignited stars are scattered outwards, symmetrically or randomly as the design calls for. The composition and size of the stars determines key aspects of the effect, such as colour, intensity, length of effect, etc.
The manufacture of stars is an art and a science, and requires a real focus on safe work practices as the occasional fireworks factory catastrophe demonstrates. Such a factory is normally a high security operation, with alarms and barbed wire fences around it. Electricity of any type is the biggest safety concern, so usually all electrical outlets are located outside the building, workers wear non-static cotton clothing, and are fitted with anti-static devices. If possible, work is done by hand rather than machine, the buildings are heated with radiators rather than static-producing hot air, and the factories usually shut down if an electrical storm is expected.
Stars contain five basic ingredients: a fuel, an oxidizer, a chlorine donor (which helps the flame burn brighter), a colour-producing substance, and a binder (usually dextrin). Black powder usually supplies the fuel, oxidizer and chlorine donor, while the colours come from metal salts (detailed below). All these raw ingredients are stored in a locked, controlled area, inside many barrels, each containing a specific chemical in powder form. The process is very much like cooking, and a recipe for each type of star is carefully followed – surprises are not desirable with fireworks. Ingredients are carefully weighed and brass sieves remove lumps (brass doesn’t produce sparks). The various quantities of chemical powders are mixed together with water to form a dough, which is shaped into large loaves of around 15 Kg. The loaves are then sliced carefully into little cubes, and dusted with black powder.
Breaks
Once dry, the stars are ready to be packed into the break’s cardboard tube. A temporary tube of smaller diameter than the break is inserted into the middle of the break. The stars are gently packed in the space between the two tubes. Once this is done black powder is poured into both the outer ring and the inner temporary tube, which is then usually removed. This leaves a central core of packed black powder surrounded by a matrix of stars and black powder in the interstitial gaps (to use some familiar terminology). Finally, a paper cap is secured at the top to create a seal.
The next step, known as spiking, is to tightly wind string around the entire length of the cardboard break cylinder. Alternatively, instead of cardboard a plastic or thicker cardboard tube might be used so that spiking is unnecessary. Regardless, the cylinder’s walls need just the right amount of structural rigidity to withstand launch stresses, but not too much so that the break won’t rupture and explode as desired. Once spiking is complete, the time-delay fuse is inserted, and the entire break is wrapped with paste-soaked paper. Once dry it is ready to be assembled with the other components of the firework. With each break designed for a certain effect, including sound, fireworks designers have all the modular components at hand to create the highly entertaining combination of effects that we enjoy.
Sounds
It is speculated that fireworks started in early China when people noticed that burning bamboo sometimes caused the sealed segments to explode. At any rate, the early Chinese fireworks were simple mechanisms designed only to produce a loud bang, ostensibly to scare away evil spirits. This was achieved by packing a segment of bamboo with gunpowder and lighting a fuse that was inserted into the powder chamber. These early fireworks were of the stationary, ground-based variety such as shown in Figure 1, and then evolved over the centuries to include more sophisticated aerial displays with the warfare-driven development of rocket and mortar technology. Nowadays, instead of single purpose bangers, various noise-producing effects are inserted into fireworks along with other, visual effects. Sound, however, is still a very big part of a fireworks show, especially when used to create a climactic grand finale effect.
Bang
The basic concept to create a bang has remained unchanged over the centuries. An explosive is tightly packed inside a sealed chamber, and when ignited, creates a high amplitude sound wave as the chamber walls rupture. For sound effects, instead of black powder a flash powder is used, typically potassium chlorate or perchlorate, mixed with sulfur and aluminum powder. These flash powders create short, sharp, loud bangs.
Crackle
A crackling sound is created by the combustion of granules made of a mix of either bismuth trioxide (Bi2O3) or bismuth subcarbonate (Bi2O2(CO3)) with magnalium, which is an alloy of aluminum and magnesium. This mixture is often referred to as ‘dragon eggs’ and produces both a crackling sound and an appealing sphere of hydrangea- like ‘blooms’. I believe the crackling sound is created by the rapid expansion of small pockets of vapour, essentially producing a barrage of small, random, high frequency shock waves, but I could be wrong.
Hummer
A very interesting humming sound is created when a break ejects a small, specially designed thick-walled tube filled with black powder which has been ignited. A small hole would have been drilled at one end of the tube, and this hole is the only escape route for the expanding gas created by the combustion occurring inside. This resulting jet of escaping gas causes the tube to spin rapidly, and creates a high frequency sound. The sound source alternately moves away and toward the listener, and this rapidly alternating Doppler effect creates the humming sound, which is complemented by a whizzing sound caused by friction between the spinning tube and the air it is rushing through.
Whistle
I have found two explanations for this effect. The first claims that burning gases resonate within the tube they are in, and the descending frequency is a result of the increasing distance between the end of the tube and the location of combustion. I suspect this explanation is overly simplistic and wrong or incomplete,
because resonant waves on the scale of a fireworks tube would be much lower frequency than the piercing whistle being produced. The other explanation is that the chosen fuels tend to strobe at an extremely high frequency during combustion, and the resulting pulses of gas being shot out create high frequency sound waves. Aromatic organic compounds are used as the fuel, usually gallic acid (C7H6O5), salicylic acid (C7H6O3) or benzoic acid salts (NaC7H5O2), with potassium perchlorate (KClO4) as the oxidizer. The descending whistle would seem to me to be a simple Doppler effect due to the firework moving away from the listener.
Colours
(Image from 'Notoriousmeow' via Imgur)
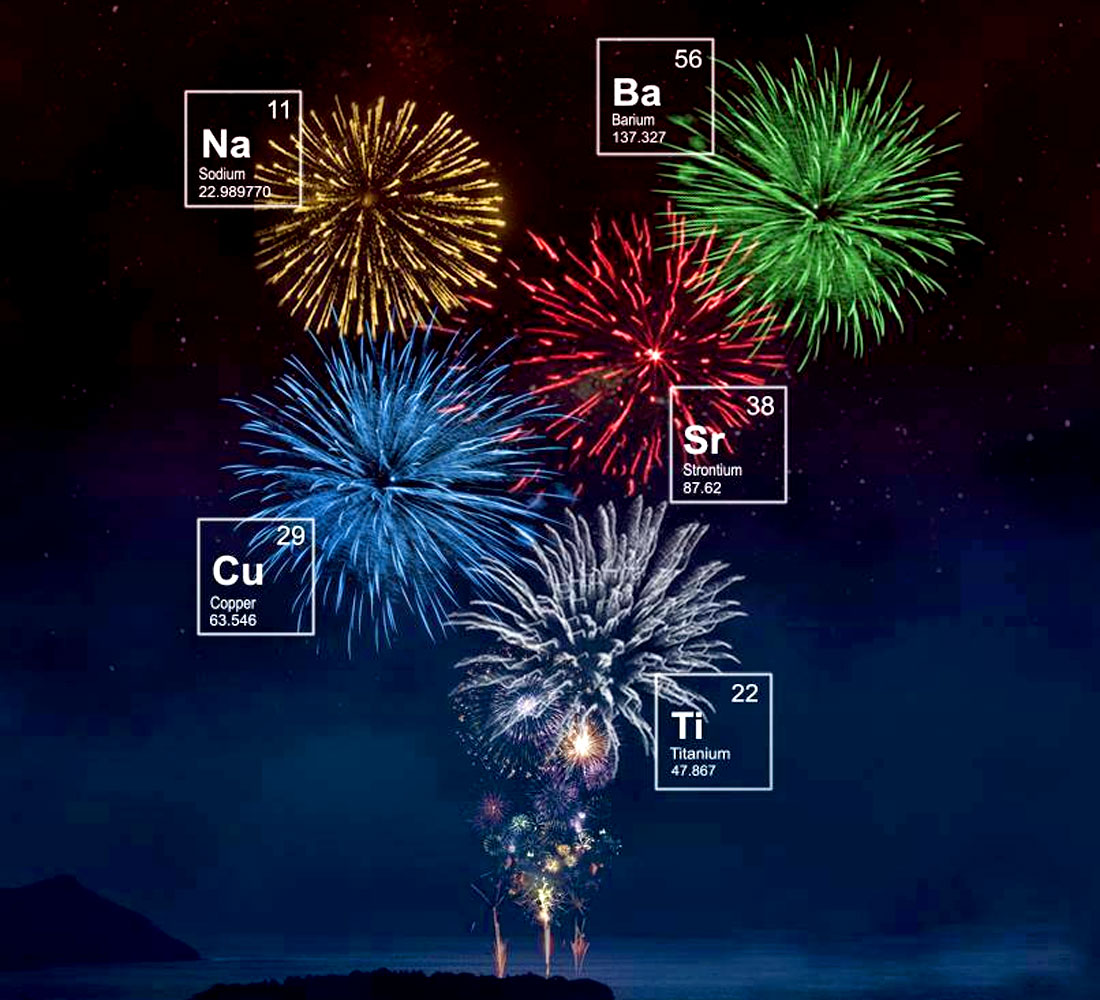
The colours seen in fireworks are created by the combustion of metal salts, as illustrated beautifully in Figure 3. Any colour of the rainbow can be chosen:
- Intense red – strontium carbonate (SrCO3)
- Medium red – lithium carbonate (Li2CO3) or lithium chloride (LiCl)
- Orange – calcium chloride (CaCl2)
- Yellow – sodium nitrate (NaNO3)
- Green – barium chloride (BaCl2)
- Blue – copper chloride (CuCl2)
- Indigo – cesium nitrate (CsNO3)
- Purple – strontium carbonate (SrCO3) + copper chloride (CuCl2)
- Violet – potassium nitrate (KNO3)
- Violet red – rubidium nitrate (RbNO3)
- White/silver sparks – powder/flakes of titanium (Ti), aluminum (Al), beryllium (Bi), or magnesium (Mg)
- Gold – charcoal (coarse carbon particles), iron (Fe) or lampblack (very fine carbon particles)
As described above, exact amounts of each chemical salt are put into a star mix, to achieve the desired colour. Then, when burned, each metal salt gives off light confined to quite a narrow spectral band.
The fine, glittery effect that you sometimes will see is usually created by burning antimony trisulfide (Sb2S3). Brighter, longer lasting white or silver sparkles are more often created by burning flakes of titanium, magnesium or aluminum; the larger the flakes the longer they last. Exceptionally bright white stars are created by burning aluminum, or magnalium.
Smoke
Creating smoke is an art in and of itself. As with stars, the secrets are entirely in the chemistry. Basic smokes usually contain some form of zinc, an oxidizer, and a few other ingredients that determine colour, thickness, duration, etc. Any imaginable colour can be added to a smoke. To get an idea of the huge range of smoke ingredients available, not to mention all other firework ingredients, check out any online store selling the raw materials that go into creating the fireworks. The website I personally have been looking at is cannonfuse.com and there you can find a collection of information if you click on ‘Pyrotechnic Info’.
Effects
Words can’t do justice to the breathtaking variety of firework effects. I found one website, keystonefireworks.com, that has a very nice video clip lexicon of 15 common types of fireworks effects, i.e. brocade, chrysanthemum, comet, crossette, etc. It makes one realise that show designers really must master a vocabulary of effects before putting together a professional display. The art of fireworks is really evident in the incredible range of effects that are achieved through variations of the basic methods I’ve described above.
To end on a personal note, I must admit I am not a big fan of fireworks. I put them in the same category as parades, because they involve a lot of fuss and bother about nothing, big crowds and standing for hours on end. But, every now and again, a fireworks display surprises me somehow. There must be something about the combination of sounds and effects that strikes a chord in me. In those rare moments, I do find myself gawking upwards in amazement, oohing and aahing with the best of them. Perhaps with some advances in science I’ll still be around in 2067 to gawk at the 200th Canada Day fireworks!










Share This Column