This month I’m going to look at glues, or adhesives as they are also known. Everybody understands these terms – they refer to substances, liquid or viscous, used to bind two objects or materials together. Writing this article underscored for me how very interested I am in ancient history, the older the better. Glues have been around since time immemorial and are definitely one of the earliest forms of technology used by hominids – to help attach a stone tool to a wooden handle for example. I would be more than happy to devote an entire article to ancient adhesives, but I’m not going to, because the modern adhesives developed over the last hundred years or so are very, very interesting. So instead I will focus on modern adhesives, and try to keep the historical content to a minimum. In terms of organization, the first section will look at how glues bond to a material, the second will describe the ways in which glues harden, and then various types of adhesives will be discussed, under the following headings: plant-based, animal-based, and synthetics.
How glues bond
Two properties determine a glue’s effectiveness – its adhesiveness and its cohesiveness. The former refers to its ability to stick to the materials it is binding together, and the latter refers to its ability to stick to itself. If the glue in a joint separates from one or both of the objects it is holding together, that is an adhesive failure; if the glue in the joint itself comes apart, that is a cohesive failure. An example of a substance with good adhesiveness but poor cohesiveness is tar – things stuck together with tar can usually be easily separated, but the tar itself stays stuck to both objects.
Many glues have quite incredible cohesiveness and tend to experience adhesive failure, but this usually happens because an inappropriate glue for the task at hand was chosen. Early in my home handyman career I thought epoxy glue was the answer to everything (including mending clothes!), similar to the way the guy on the Red Green Show feels about duct tape. However, I soon found out that epoxy easily separates from some substances. So the adhesive property of a glue in relation to what is being glued together is really at the heart of how glues work. As with many areas of science, there exists active debate about what is actually going when one substance adheres to another, which I suppose is part of the reason science is so exciting – it’s an area of dynamic learning. Several mechanisms can be involved in adhesion, most importantly mechanical bonding, chemical bonding and Van der Waals forces. In some joints there may be a small amount of electrostatic attraction (i.e. ionic bonding) between glue and material, and perhaps some covalent bonding (the sharing of electrons between atoms) but my understanding is that these are second order factors, and I won’t discuss them any further.
Mechanical bonding
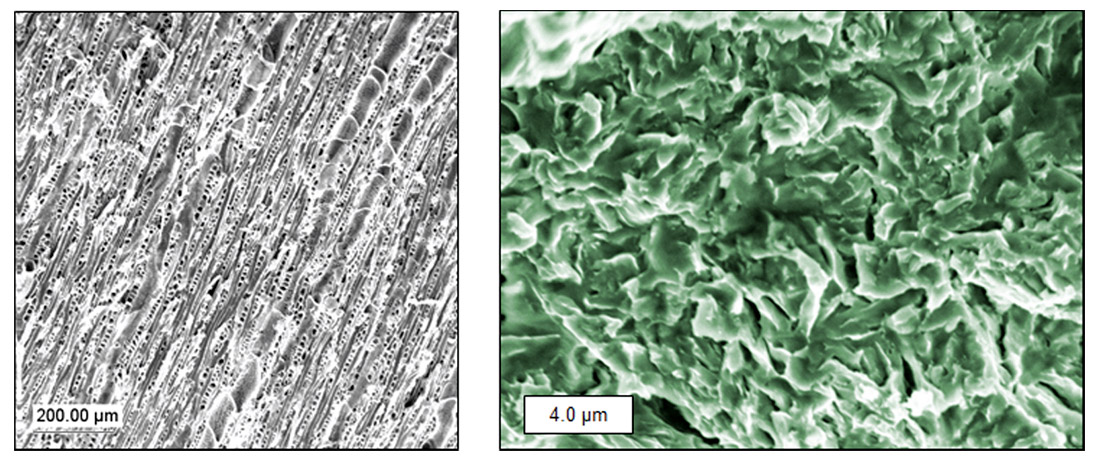
This type of bonding is easy to imagine. At a microscopic level materials have all sorts of gaps and imperfections, something we as geoscientists know very well – the oil and gas industry relies on rock porosity. Because glues are liquid, they will tend to flow into all these little surface cracks and gaps, and once hardened the glue is essentially anchored to the material in a physical sense. Looking at the magnified images of wood and metal in Fig. 1 it is easy to see that if a glue could flow into all that pore space and harden, then the effect would not be unlike millions of wall anchors in drywall.
Chemical bonding
Again, this one is fairly easy to understand. Some glues actually break down the molecular structure of the material(s) being bonded and mix together at a chemical level, and this mixture then hardens. A great example of this is hobby glue used to build plastic models. Back in the day when I was building little cars (and getting glue all over my fingers and no doubt suffering brain damage from the fumes) what was used was polystyrene cement, which dissolves plastic. Nowadays there is a broader range of model glues employing chemical bonding, and they vary in the types of plastics they dissolve, and are generally safer to use and more environmentally friendly.
Van der Waals forces
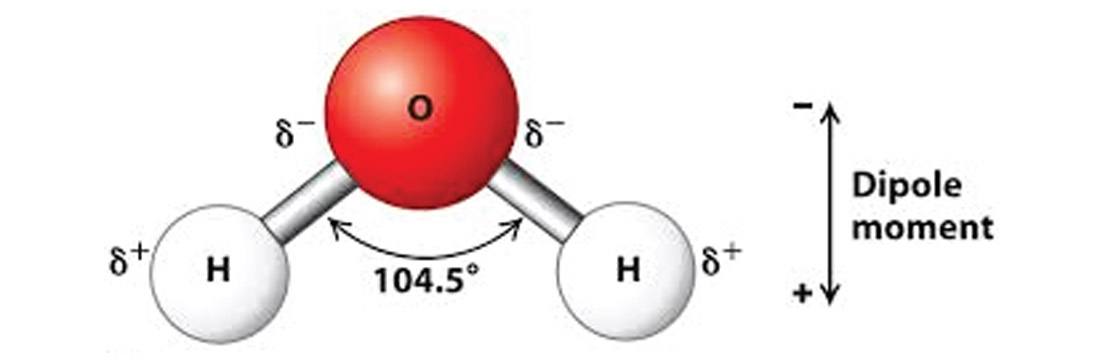
These forces are essentially electrical, at a molecular level. Many molecules are slightly polarized, because there is some inherent asymmetry in their structures. For example water molecules (Figure 2), because the two hydrogen atoms are off to one side at an angle, display a dipole moment as shown. If a glue is liquid enough to get in really close contact to the material it is adhering to, then the positive poles of molecules in the glue will be attracted to negatives in the material being bonded, and vice versa. These electrical attractions are minute, but when multiplied by the millions (or billions?) of molecules in contact within a glued joint, then their sum can be quite powerful.
I prefer to think of the primary source of adhesiveness as being a combination of mechanical bonding at a small scale, and Van der Waals forces at an even smaller scale, with sometimes a certain amount of chemical bonding being thrown in as well. In some cases what is going on is not easily categorized as I have done above. For example, if a glue has long molecules whose chains can penetrate into the molecular structure of the material being bonded, is that mechanical or chemical bonding?
Slightly off topic, but I’m fascinated by the adhesiveness of gecko feet. I’ve seen that explained by a mechanical Velcro-like mechanism involving thousands of tiny hairs on their feet “hooking” on to a surface’s imperfections, but I’ve also seen it explained by Van der Waals forces between the hairs and the surface’s molecules. In the end I’m not sure it really matters – I believe having different descriptions of the same effect actually increases understanding, because words are never truth, they are just clumsy attempts to describe it. (Wow, that was quite a metaphysical thought!)
How glues harden
All this business about adhesiveness is fine, but how do glues only bond upon application? A glue is only useful if it can reliably be transformed from a liquid (that preferably can be stored, handled, and so on) into a hard or semi-hard bonding agent with predictable adhesive and cohesive properties. Different glues achieve this in different ways – chemical reaction, cooling, exposure to an external energy source, drying, and pressure/contact.
Drying
This type of glue has probably been around the longest; many natural glues contain enough volume of water that they are liquid, and then as the water evaporates (or is absorbed by the neighbouring material) the glue hardens and bonds. Nowadays drying glues fall into two types – solvents and polymer dispersions. It’s a fine distinction between the two. With the former category the glue substance is dissolved in the solvent, while with the latter it is dispersed in the liquid but not dissolved. Another way to look at it is that a solvent glue is homogenous while a dispersion is heterogeneous. If you’ve ever wondered what an emulsion is, it’s the same as a dispersion, but instead of a solid being dispersed within a liquid, it’s a liquid held in suspension within another liquid. Quicker drying glues use solvents or dispersants that evaporate or absorb more readily than water (e.g. alcohol), but lately there has been a swing back towards water-based glues because they are more environmentally friendly. White glue is a classic solvent adhesive and thinking of it reminds me of early school days. I was always baffled why some kids would eat white glue, and still am. What happened to those kids? Did they grow up to be normal adults?
Cooling
These glues exploit the simple natural law that substances are liquid at some higher temperature and solid at some lower temperature. So many types of glues are liquefied by heating, applied, and then as they cool they harden. A common example is that of glue gun glue, also known as hot melt adhesive (HMA). HMA’s are typically made up of a base material, usually some kind of polymer such as ethylenevinyl acetate (EVAS) or polyolefins, plus additives that combined give the desired bond strength, melt temperature, flexibility, temperature range of effectiveness, viscosity, crystallization temperature and so on. A fuller explanation of what exactly polymers are will come later.
Chemical reaction
I’ve already mentioned an example of a glue that relies on chemical reaction – my favourite, epoxy. Glues like this come packaged as two or more separate components, which are mixed together when the glue is to be used. Typically the components contain different types of polymer which on their own are non-adhesive, but when mixed together combine chemically to form either acrylic, urethane, or epoxy. They are really part of the drying category, as once mixed they behave just like a solvent or polymer dispersion glue. I believe that an advantage of these types of glue is that the trouble of creating a single liquid that can be contained and stored until application is avoided, and likely more aggressive (in terms of adhesion and set time) adhesives can be created without requiring all the extra ingredients used to achieve those desired pre-application properties.
I should mention cement or mortar as a type of adhesive which does not fit into the categories I use below (plant-based, animal-based, synthetic). The ancient Greeks were the first to experiment with mixtures of sand, lime and water, but it was the Romans who really perfected the first cement capable of bonding stone with high levels of adhesion – they combined lime, volcanic ash from Mt. Vesuvius, and water to create pozzolanic mortar. It is said that the construction of the Colosseum would have been impossible without this mortar.
External energy source
It could be argued that these kinds of glues also rely on chemical reaction, because that is exactly what happens. Instead of the chemical reaction being caused by the combination of two reactants, it is facilitated by the introduction of some type of energy. Just last week I had a broken tooth fixed, and as I lay there with my mouth open I was thinking that what the dentist was using would be a great example of this type of adhesive. The material used to replace the tooth enamel is a composite resin designed to possess the desired properties of colour, malleability, cure time, adhesion, ultimate hardness, and all sorts of other aspects that the dental industry has improved over the decades since these substances first started replacing metal amalgam (mercury alloy) fillings back in the early 1960’s. The latest technology employs an LED light source in which a gallium nitride semiconductor emits intense blue light. This light cures the resins in the dental composite within 3 to 5 seconds, to a depth of 2-3 mm. This is why a dentist will build up a filling with several layers, because obviously you don’t want a thicker filling to contain a soft core.
Pressure / contact
Contact cement is a good example of this type of glue. Here the drying occurs before the two surfaces are brought together. As the glue dries, it creates a hard film on the surfaces of the objects to be glued. Once dry, as soon as the surfaces are brought into contact, they begin to bond at a chemical level. There are advantages to these kinds of glues – because they dry before the joint is made, they can be used to bond non-porous materials through which there is no way for solvents to escape. They are also perfect for use in situations where clamping is difficult, as the bond is almost immediate.
Another type of adhesive that could be mentioned here is the low-tack, pressure-sensitive, re-usable glue used with the ubiquitous sticky note. Here a sparse monolayer of acrylate copolymer creates a bumpy dimpled surface with just the right amount of stickiness. When you apply pressure the copolymers form a mechanical bond with the other surface, and then they just as easily separate under tension, ready to use again and again until dirt clogs up the monolayer.
Plant-based glues
For most of human history, these and animal-based glues were all that was available. Before launching into this topic, let me touch on what a resin is. A resin is a naturally occurring hydrocarbon produced by a plant, mainly to ward off damaging insects or to attract predators that eat the damaging insects. Resins are viscous, and tend to dry into a hard substance, like amber for example. They would have been an obvious choice as an adhesive to primitive humans – one only has to touch pine sap once by mistake to realize it’s very sticky! Resins tend to be water soluble and brittle once hardened, making them less than ideal as adhesives; however, early humans discovered that additives could make resins more water resistant and flexible. For example, 70,000 year stone hand axes have been found in South Africa with traces of an adhesive with a plant gum base and an iron oxide ochre additive.
I can’t let this opportunity pass to mention Ötzi, the 5,200 year old man found preserved in a Tyrolean glacier. The analysis of his remains, including his tools, represents to me one of the most exciting scientific stories in my lifetime; one big impact of his discovery was the knowledge gained about the technology of that time and place. He carried with him flint arrows and copper tools, and the glue used was birch pitch, a resin extracted from birch bark via heating (Spindler, 1994) (Figure 3).

Over the millennia humans developed all sorts of plant-based glues using flour and other starches (i.e. dextrins, which are mixtures of polymers and glucose), as well as a huge range of resins. I suppose you could include geologically derived hydrocarbons as being plant-based, but it’s a bit of a stretch. In my very first article for the Recorder (Odyssey of Oil, Dec., 2000) I mentioned references to the use of tar in Herodotus and the Bible, as well as other prehistoric uses of oil-based substances as adhesives and sealants.
Animal-based glues
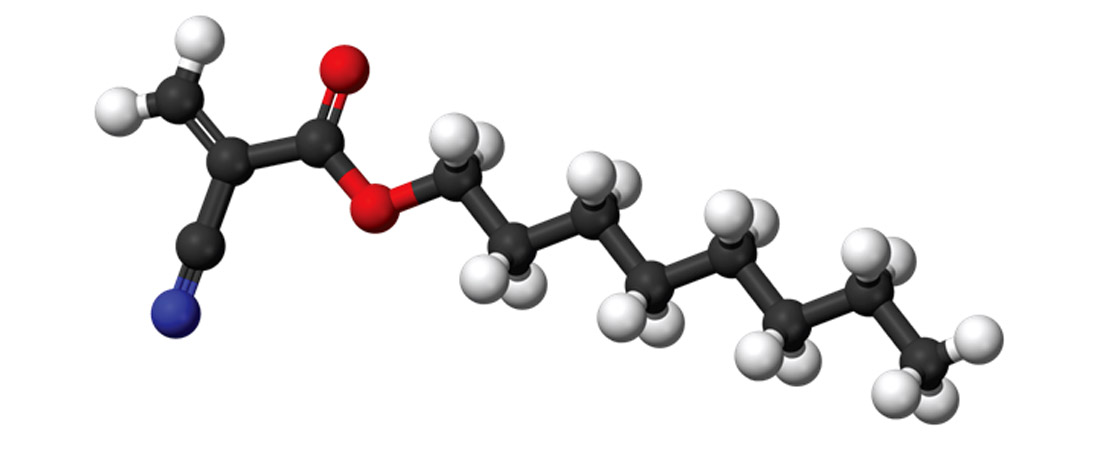
This category demands the overdue explanation of what a polymer is. A polymer is a large molecule or macromolecule with repetitive sub-units. Many are long, chain-like structures, and I believe this is why they play such a large role in adhesives – their long dangly bits make it easy to get tangled up in other substances, both in a mechanical sense and in Van der Waals forces sense. Figure 4 shows an octyl cyanoacrylate molecule, which is a polymer used as the main ingredient of a range of medical glues which provide rapid, painless closure of wounds and incisions. The term polymer applies to a huge range of substances, both man-made and natural. Natural or biopolymers include DNA, and importantly here, the proteins collagen and keratin. It is primarily these polymers that are used in animal-based glues. Collagen is the most common structural material found in animals, especially in bones and connective tissues such as ligaments. Keratins are the structural components in hair and nails. Both these polymers have been extracted and used in adhesives, which until the advent of synthetic glues were the dominant glues of choice.
Glues formed from collagen (also known as protein colloids) were historically created by hydrolysis of animal bones and connective tissue. Basically this meant throwing animal remains (especially from horses and cows) into a big vat of water and boiling the crap out of them until the collagens separated out. The semi-purified collagens could then be mixed with water or other solvents to create a glue paste. Over the centuries these methods became more sophisticated and controlled, resulting in a broad range of glues for different purposes. Fish glues are typically made from the collagens extracted from fish bones, but a particularly pure form of glue called isinglass is formed from polymers extracted from sturgeon bladders. Besides its use as a fining agent to accelerate the clarification of beer and wine, isinglass was commonly used to re-adhere paints to parchment, such as in the repair of illuminated medieval bibles.
Other types of animal proteins can be isolated and extracted for glue use, including albumen from egg whites, and casein from dairy products and blood. Included in this category would also be beeswax, used as an adhesive and sealant since ancient times.
Synthetics
As I mentioned before, there has been an explosion of synthetic adhesives over the last 100 years or so, far too many to go through one-by-one. Once you understand the basic ingredients and mechanisms at play, it is quite easy to imagine the multitude of ways glues can be custom designed for very specific purposes; a look at the glue section at a hardware store can be quite mind boggling as there are so many to choose from. So why don’t we look at a few common glues before wrapping up for this month?
Epoxy
There are so many different types of epoxy glues available that it’s pointless to try to cover them all. Figure 5 shows a very common type of epoxy glue, with the two components clearly obvious. When these two are mixed together, they will start to cure, and form a hard interlinked structure that is a powerful adhesive. Commonly one component is known as the resin, and the other the hardener. The resin is typically some kind of polyepoxide, and the hardener or curing agent something like an amine, alcohol, thiol, phenol or acid. Epoxies can be made with a huge variety of properties (colour, temperature range, resistance to chemicals, etc.) so they are used in many, many industrial and commercial applications.

Polyvinyl acetate (PVA)
This class of synthetic glues includes Elmer’s white glue and common carpenter’s glue. Their popularity is due to the fact that they are good at binding to porous surfaces such as paper and wood, and are non-acidic in nature and so relatively benign in terms of damaging the materials they are binding together. The chemical formula for polyvinyl acetate is (C4H6O2)n and it is formed by polymerizing vinyl acetate monomers (a monomer is just a molecule that can bind to another molecule to form a polymer). Just typing out these chemical names makes me even more mystified why kids eat hardened white glue.
Cyanoacrylates
These glues, including brand names “Super Glue” and “Krazy Glue” had semi-mythical status when I was growing up. There always seemed to be urban myths (which most of us children totally believed) of people gluing their eyelids open, or bonding their mothers’ derrieres to the toilet seat. Later in life I was led to believe that they were originally developed during the Vietnam War as medical glues to quickly close wounds in the battle field. There is an element of truth to this as some cyanoacrylates are now used as medical adhesives (Figure 4), but the reality is that scientists trying to develop better gun sights during WWII stumbled upon a substance that stuck to everything very quickly, but did not commercialize it as a glue. Kodak rediscovered cyanoacrylates in the mid-50’s, and came out with a commercial version in 1958.
The cyanoacrylates in liquid form in the tube are made up of acrylate monomers. As soon as they come into contact with even small amounts of water they quickly polymerize and cure; when applied, the humidity in the air is sufficient for curing. Their main claim to fame is their extremely fast set time, water resistance, and adhesive strength, but only to certain substances, mainly porous ones. They are not good at bonding smooth materials such as glass, they have a short shelf life, and they have poor shearing strength.
Rubber cement
I wanted to mention this kind of glue, just because rubber is such a cool material. I’ve actually considered doing an entire article on rubber, because it has so many unique properties. The polymers that make up rubber as we know it are polyisoprenes, and are known as elastomers. This is because they can be stretched, but then return to their original shape. The polyisoprene chains are a mix of really long linear and “wrinkled” chains, with the latter dominating. I wanted to show an image of a polyisoprene molecule, but the best ones are stock photos meaning I’d have to pay to use them – just Google it and you’ll see how wrinkled up they are. When stretched the wrinkled chains elongate, but as soon as the tension is removed they return to their wrinkled shapes. In glues such as rubber cement the polyisoprenes are held in a solvent such as water, acetone, hexane, heptane or toluene, and they set when the solvent evaporates. The beauty of rubber glues is that they form a strong bond that retains the unique flexible, stretchy properties of rubber.
I regret not being able to touch on the many other glues available and their unique properties and histories, but space is limited as is the mental adhesion of readers! I also need to get cracking on next month’s article, which promises to be an interesting foray into the social sciences.










Share This Column