Quite frequently my 12-year old son asks me questions that have me opening my mouth to answer only to realise that I don’t have a clue! This happened not long ago when I explained to him that companies were mainly drilling gas wells that provided natural gas liquids (NGL’s). When he asked what the NGL’s were, I stammered out something lame like, “Butane...propane...a bunch of gases that end with ‘ane’.” Of course that left me feeling quite inadequate, so I have researched NGL’s, and provide an overview of that information for this month’s article. I try to be rigorous with references, but I used a lot of different Wikipedia pages – one just leads to another! Let’s just say Wikipedia is the primary source for this article (including the little molecular graphics), to avoid a huge list of references.
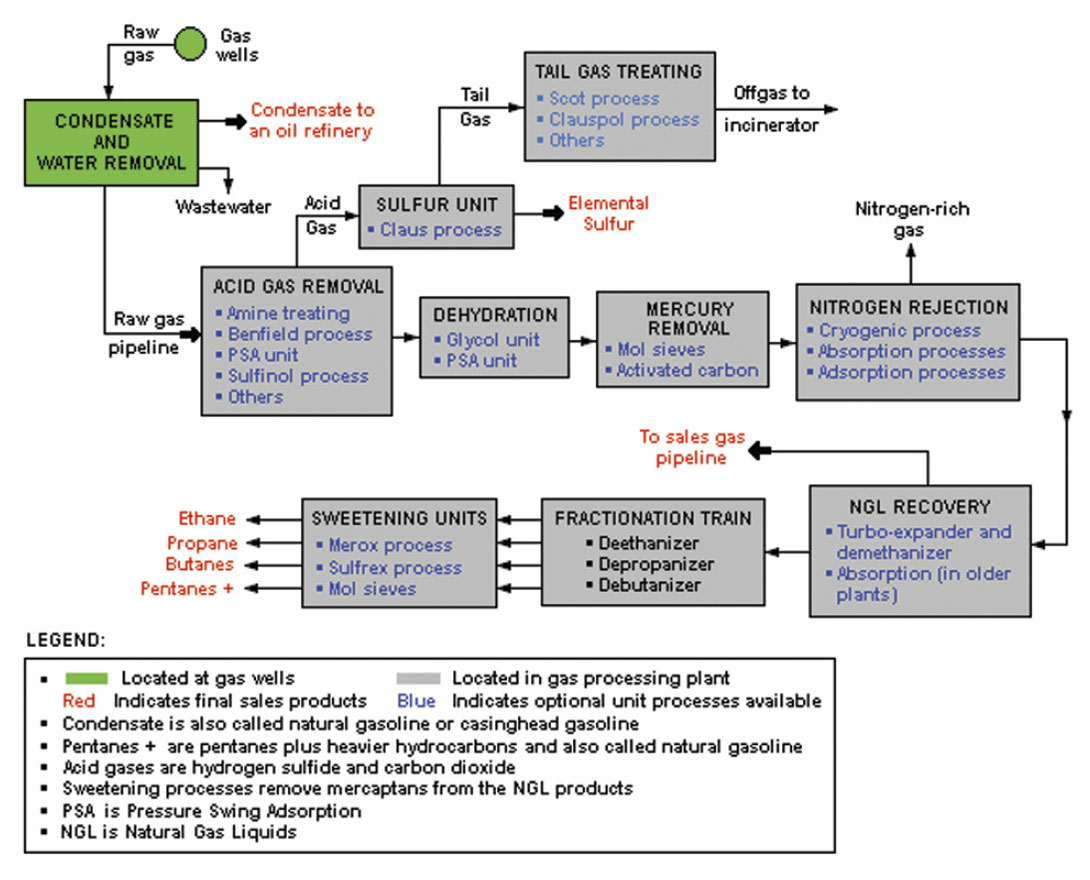
Besides the methane (CH4) which constitutes 80% to 95% of natural gas, the NGL’s primarily include ethane (C2H6), propane (C3H8), normal butane (n-C4H10), isobutane (i-C4H10), pentanes, plus some other hydrocarbons of even higher molecular weight. These are the NGL’s with some commercial value, but natural gas can also contain other gases such as carbon dioxide (CO2), hydrogen sulfide (H2S), mercaptans such as methanethiol (CH3SH) and ethanethiol (C2H5SH), and of course nitrogen (N2) and helium (He) (Wikimedia Foundation, Inc., 2012). A shortage of helium has been reported in the news lately, so it has actually gone up in value. Obviously the gas coming out of the ground is complex and must be processed, to isolate the valuable components and dispose of the worthless and dangerous ones – a complex and highly interesting topic in itself. Wikipedia provides an excellent diagram that outlines the key steps in the process (Fig. 1) and we’ll leave it at that. Here are some highlights regarding the commercially valuable NGL’s:
Ethane
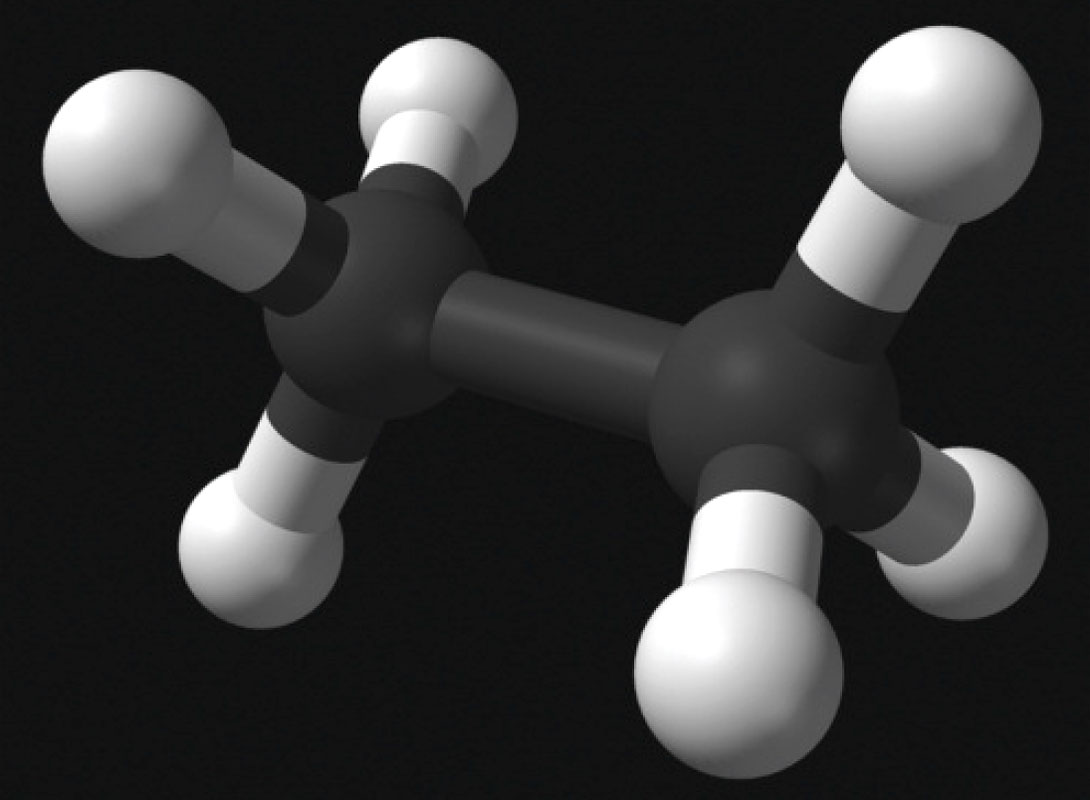
Ethane (C2H6) is a continuous-chain alkane, and usually makes up between 1% to 6% of natural gas (Wikimedia Foundation, Inc., 2012). Its primary use is as a feedstock in the production of ethylene, via high temperature steam cracking (C2H6 → C2H4). Cracking is the process whereby complex hydrocarbons are broken down into short-chain hydrocarbons such as simpler alkanes, and alkenes. Ethylene has a huge variety of uses, including as a fruit ripener (it is a natural plant hormone) and in the production of the most widely used plastic, polyethylene. (Wikimedia Foundation, Inc., 2012).
Propane
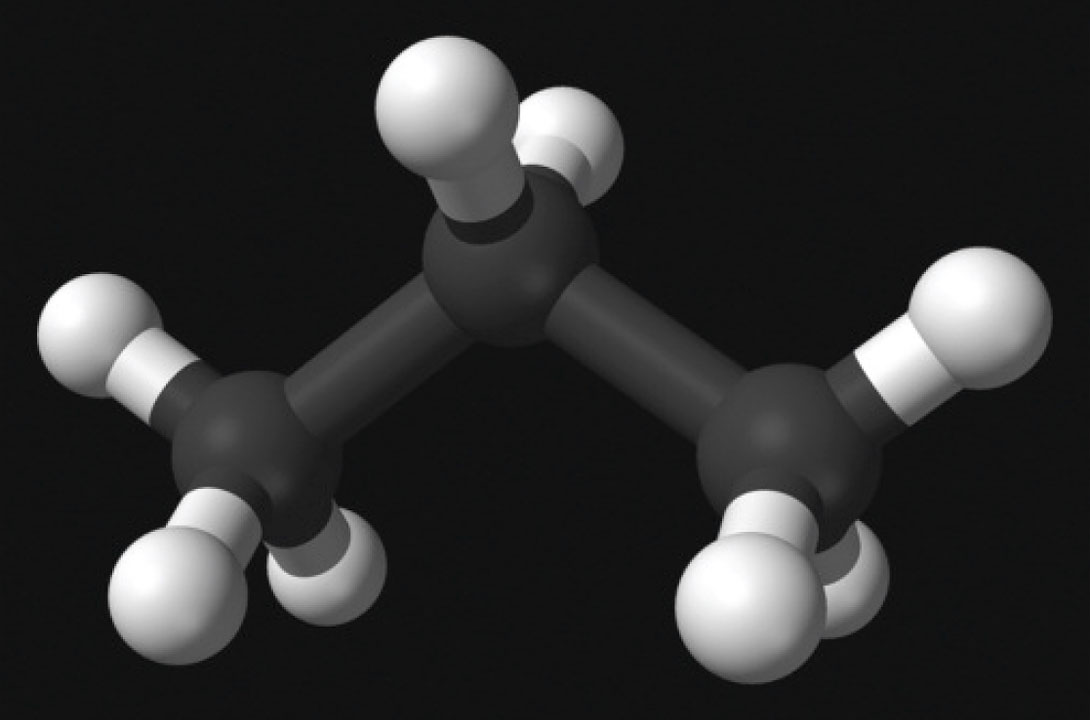
Propane (C3H8) is a colourless, gaseous alkane and is primarily used as fuel – in cars, camp stoves, lighters, etc. Its commercial use exploded (metaphorically speaking!) quite recently, after the patent for production of liquid propane (via compression and cooling) was awarded in the 1920’s (Wikimedia Foundation, Inc., 2012). It is attractive as a fuel because it becomes liquid at moderate pressures easily maintained through safe, affordable and transportable containers such those used with BBQ’s. An obvious downside is that propane is heavier than air; hence dangerous situations are created if propane leaks and collects in low lying areas, like underground parkades. This brings to mind a previous Science Break article, “Africa’s deadly lakes” concerning heavy gases. (Kuhn, 2009).
Normal Butane
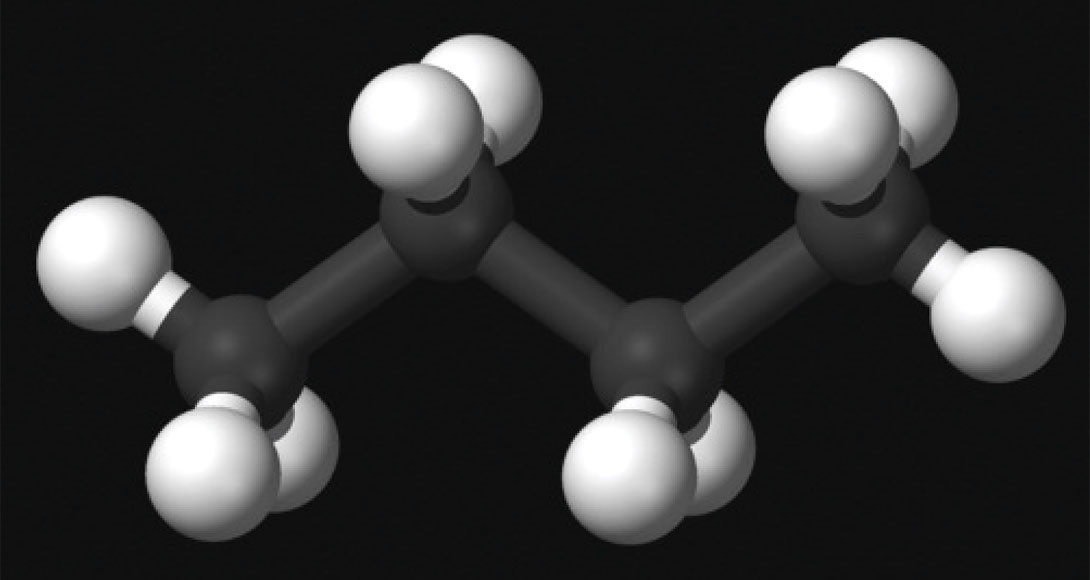
This is the unbranched form of butane (n-C4H10), where the four carbon atoms are connected in a continuous chain. It has numerous uses – in lighters, camp stoves etc., as a propellant for spray cans, and as an ozone-friendly refrigerant replacing halomethanes. Interestingly, it is the cause of over half of propellant related deaths. If accidentally (or on purpose?) sprayed into a human throat, as it depressurises it cools rapidly to about -20°C and the larynx can go into spasms causing suffocation.
Butane is very commonly mixed with propane when used as a fuel. Why? Propane’s high vapour pressure requires a heavy canister to safely contain it. Butane on the other hand can be safely contained in a much lighter container, but its lower vapour pressure means as the ambient temperature drops, it is harder to ignite. So for backpacking purposes the blend offers the best of both worlds – lightweight, easy to light (Wikimedia Foundation, Inc., 2012).
Isobutane
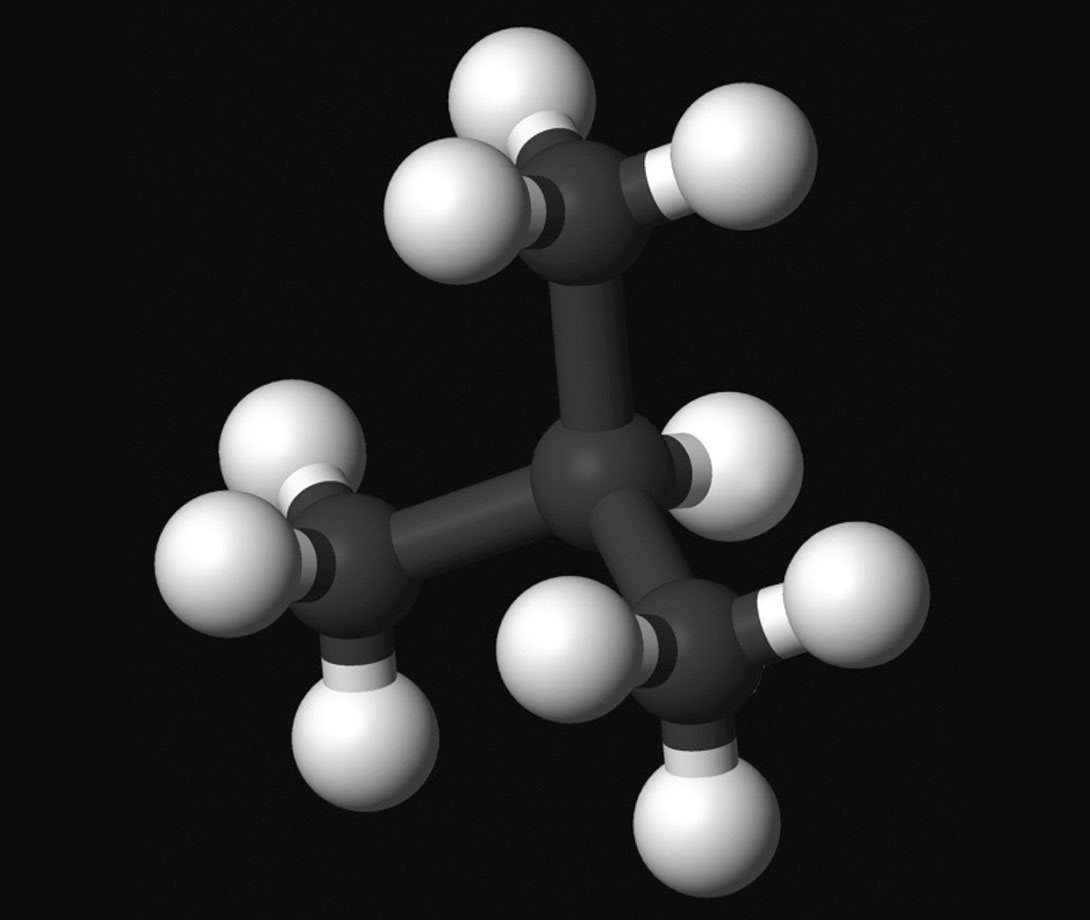
Isobutane (i-C4H10) differs from butane in that three of the carbon atoms are connected to the fourth by branches. In addition to being used for all the same purposes as butane, it is also very commonly used to upgrade petroleum via alkylation. Typically the isobutane is alkylated with a mixture of butene and propene (lower molecular weight forms of butane and propane), with hydro fluoric or sulphuric acid acting as the catalyst (Wikimedia Foundation, Inc., 2012). What happens in the alkylation process is that the double carbon bonds within the isobutane are broken and replaced with single bonds with elements within the butenepropene mix, and the result, called alkylate, burns really clean and has anti-knock properties. It is then blended with gas to produce the different grades we get at the pump. I may have the details wrong because my chemistry is weak, but you get the picture...
Pentane
There are a number of different pentanes of increasing complexity (e.g. isopentane, neopentane), but the term usually refers to C5H12. It has properties similar to butane, and is mostly used as a solvent and as a minor component within fuel mixtures. (Wikimedia Foundation, Inc., 2012).
Natural Gasoline / Condensate
This liquid is a mix of hydrocarbons (mainly pentanes) whose vapor pressure falls between that of drip gas and liquefied petroleum gas, its boiling point is similar to that of gasoline, and its gravity is around 80 API. Its octane content is too low to be used directly as a substitute for refined gasoline, but it is mostly used to blend with ethanol to produce a gasoline alternative for flex-fuel vehicles (Wikimedia Foundation, Inc., 2012).
Sulfur
Natural gas which contains more than about 5.7 mg of hydrogen sulfide (H2S) per cubic metre is referred to as sour; natural gas with negligible amounts of H2S is called sweet gas. Those terms should make it clear that the sulfur is considered undesirable! The H2S is toxic, and combined with water makes sour gas highly corrosive. The H2S is removed using the Claus process, and stockpiled as elemental sulfur (yellow mounds of it are visible at the gas plant north of Calgary Airport). It is used for various purposes, such as making fertilizers and as an industrial feedstock (Wikimedia Foundation, Inc., 2012).
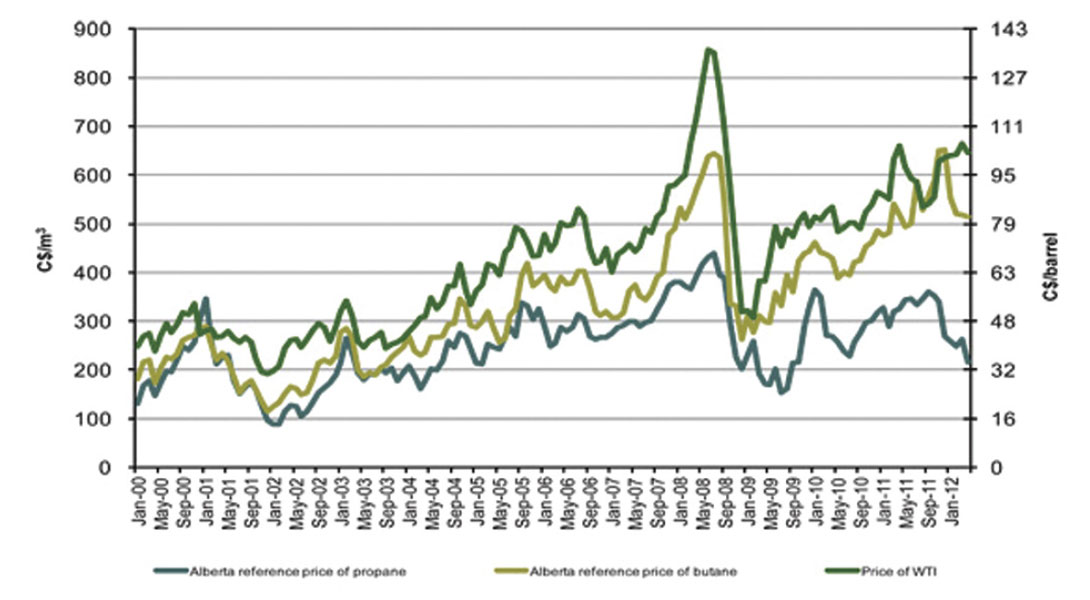
A sad (at least to most readers of this article) footnote is that prices for NGL’s seem to be dropping as the boom in shale gas and focus on liquids-rich gas has pushed up supplies; see Figure 2 (National Energy Board, 2012).
References
BP. (2012). What is NGL? Retrieved September 25, 2012, from BP: http://www.bp.com/sectiongenericarticle.do?categoryId=3050079&contentId=30 50155
Kuhn, O. (2009, May). Africa's deadly lakes. CSEG RECORDER, pp. 56-59.
National Energy Board. (2012, July 13). Natural Gas Liquids – How Canadian Markets Work. Retrieved September 28, 2012, from National Energy Board: http://www.neb-one.gc.ca/clf-nsi/rnrgynfmtn/prcng/ntrlgslqd/cndnmrkt-eng.html
Wikimedia Foundation, Inc. [Numerous pages] Retrieved September, 2012, from Wikipedia: http://en.wikipedia.org/wiki/










Share This Column